Explain why the hydrogen migration shown in reaction (1) occurs readily and why the very similar migration
Question:
Explain why the hydrogen migration shown in reaction (1) occurs readily and why the very similar migration shown in (2) does not take place even under forcing conditions. (The asterisked carbons indicate a carbon isotope present so that the rearrangement can be detected.)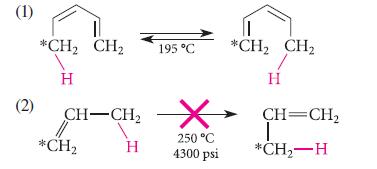
Transcribed Image Text:
(1) (2) *CH₂ CH₂ H CH-CH₂ // *CH₂ H 195 °C 250 °C 4300 psi *CH₂ CH₂ T H CH=CH₂ T *CH₂-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Reaction 1 occurs readily because it is a 15 sigmatropic rea...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
AMECO Relocation Decision Problem Agricultural Machinery Exporters Company (AMECO), which has its headquarters in the UK, is considering opening a manufacturing plant in an overseas country and...
-
In the discussion on stable storage, it was shown that the disk can be recovered to a consistent state (a write either completes or does not take place at all) if a CPU crash occurs during a write....
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The administrator of Hope Hospital has been asked to perform an activity analysis of the emergency room (ER). The ER activities include cost of quality and other patient care activities. The lab...
-
Sterling Steel Inc. purchased a new stamping machine at the beginning of the year at a cost of $580,000. The estimated residual value was $60,000. Assume that the estimated useful life was five...
-
As a jewelry store manager, you want to offer credit, with interest on outstanding balances paid monthly. To carry receivables, you must borrow funds from your bank at a nominal 9%, monthly...
-
(2) If you worked for the organization concerned, which organizational audiences might require an account of the change to be constructed?
-
Kimberly Manis, an architect, organized Manis Architects on January 1, 2016. During the month, Manis Architects completed the following transactions: a. Issued common stock to Kimberly Manis in...
-
Amazon Beverages produces and bottles a line of soft drinks using exotic frults from Latin America and Asia. The manufacturing process entalls mixing and adding Juices and coloring Ingredients at the...
-
(a) What allowed and reasonable sigmatropic reaction(s) can account for the following transformation? (b) What product(s) are expected from a similar reaction of 2,3-dimethyl-1,3- cyclopentadiene?...
-
Classify the following sigmatropic reactions with bracketed numbers. (a) D. (b) (c) Ph :- I D. S Ph
-
Refer to the information about Adarmes Adventures given in Exercise 6-4. Alexis King chose to prepare a static budget based on sales of 3,000 canoes. Actual sales were 3,100 canoes at a price of $850...
-
int rFibNum(int a, int b, int n) { if(n == 1) return a; else if( n == 2) return b; else return rFibNum(a,b, n-1) + rFibNum(a, b, n-2); } In the code above; a) how many base cases are there? b) what...
-
Watch the Super Nanny (i.e., Jo Frost) episode "The Orm Family" (Season 1, Episode 3) and answer the following questions. Unless otherwise specified, your answers should focus on Declan (the 3 year...
-
2. Suppose Ford officials were asked to justify their decision. What moral principles do you think they would invoke? Assess Ford's handling of the Pinto from the perspective of each of the moral...
-
2. You have been asked to design the proto-type of an Automatic Grocery Vending Machine 10 (AGVM) for the super store. Automatic Grocery Vending Machine (AGVM) is a machine where different types of...
-
1. What does Porter's 5 Forces analysis strategy do? 2. Do most people agree Why? or disagree with this aspect Why? Here is the reference video, https://www.youtube.com/watch?v=Dfp23xSqpdk 3. What...
-
During the first month of operations ended May 31, 2016, Frost Point Fridge Company manufactured 40,000 mini refrigerators, of which 36,000 were sold. Operating data for the month are summarized as...
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
Give a general balanced reaction for The complete combustion of a cycloalkane containing one ring formula CnH2n.
-
Carv and Di Oxhide drive their family car about12,000 miles per year. Their car gets about 25 miles per gallon of gasoline. "What is the carbon footprint" (pounds of CO, released into the atmosphere)...
-
Draw a structural formula for each of the following compounds. (Several formulas may be possible in each case.) An alcohol with the molecular formula C5H10O
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


