Fatty acids containing an even number of carbon atoms are readily obtained from natural sources, but those
Question:
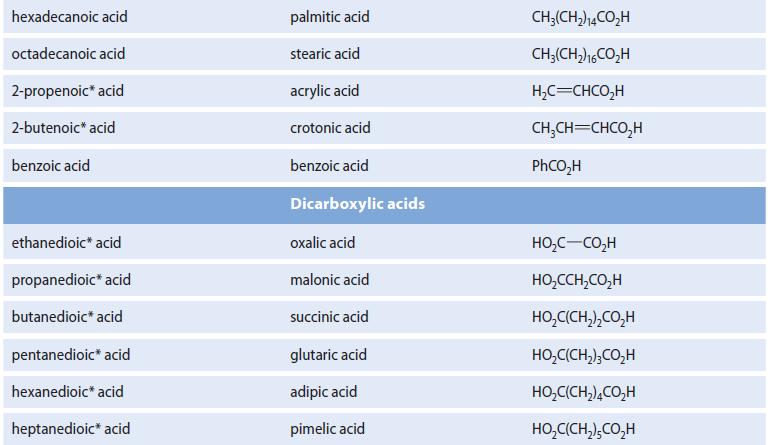
Fatty acids containing an even number of carbon atoms are readily obtained from natural sources, but those containing an odd number of carbons are relatively rare. Outline a synthesis of the rare tridecanoic acid, CH3(CH2)10CH2CO2H, from the readily available lauric acid (see Table 20.1).
Transcribed Image Text:
TABLE 20.1 Names and Structures of Some Carboxylic Acids Systematic name Common name methanoic acid ethanoic acid propanoic acid butanoic acid 2-methylpropanoic acid pentanoic acid 3-methylbutanoic acid 2,2-dimethylpropanoic acid hexanoic acid octanoic acid decanoic acid dodecanoic acid tetradecanoic acid formic acid acetic acid propionic acid butyric acid isobutyric acid valeric acid isovaleric acid pivalic acid caproic acid caprylic acid capric acid lauric acid myristic acid Structure HCO₂H CH,CO,H CH,CH,CO;H CH,CH,CH,CO,H (CH3),CHCO,H CH3(CH₂)3CO₂H (CH3),CHCH,CO,H (CH3)3CCO₂H CH₂(CH₂)4CO₂H CHJ(CH,)%CO,H CH3(CH2)gCO;H CH,(CH2)CO,H CH₂(CH₂)2CO₂H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The problem requires the synthesis of a carboxylic acid with the addition of one carbon ato...View the full answer

Answered By

Irfan Ali
I have a first class Accounting and Finance degree from a top university in the World. With 5+ years experience which spans mainly from the not for profit sector, I also have vast experience in preparing a full set of accounts for start-ups and small and medium-sized businesses. My name is Irfan Ali and I am seeking a wide range of opportunities ranging from bookkeeping, tax planning, business analysis, Content Writing, Statistic, Research Writing, financial accounting, and reporting.
4.70+
249+ Reviews
530+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A company is looking at compete in some large consumer products in China. What type of site selection issues will they be looking at when locating a new facility in China ? How would these site...
-
A fatty acid with an even number of carbon atoms is metabolized to acetyl-CoA, which can enter the citric acid cycle. A fatty acid with an odd number of carbon atoms is metabolized to acetyl-CoA and...
-
2. Consider the kinetic energy operator, - h d 2m dx (a) Show that sin x and sin 2x are eigenfunctions. (b) Show that these eigenfunctions are orthogonal.
-
Calculate the 90% confidence interval for the difference (mu1-mu2) of two population means given the following sampling results. Population 1: sample size = 19, sample mean = 20.52, sample standard...
-
The accountant for Klein Photography has posted adjusting entries (a)(e) to the following selected accounts at December 31, 2012. Requirements 1. Journalize Klein Photographys closing entries at...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
What percentage of IQ scores for rural Midwest seventh-graders are less than 100?
-
A letter in the December 1995 issue of Dell Champion Variety Puzzles stated: Ive noticed over the last several issues there have been no winners from the South in your contests. You always say that...
-
Select a publicly traded company. Answer the following questions about the Statement of Cash Flows. For example: Kroger 1. Identify the largest investing activity and the largest financing activity...
-
Identify the compound C 7 H 10 O that has an IR absorption at 1703 cm 1 and the proton NMR spectrum shown in Fig. P19.71. Figure P19.71 The NMR spectrum for Problem 19.71. The relative integrals are...
-
(a) You are the chief organic chemist for Bugs and Slugs, Inc., a firm that specializes in environmentally friendly pest control. You have been asked to design a synthesis of 4-methyl-3-heptanol, the...
-
Summarize your impression of each of the three sites, and then compare notes with your teammates. Based on the strengths and weaknesses of each site, identify four pieces of advice for a company that...
-
Ted sold his Microsoft stock for $40,000 paying a commission of $800. He purchased the stock in 2004 for $8,000 and paid commission of $200. What is the recognized gain on the sale?
-
Liquid water at 80C and at 1atm flows through a heated pipe at a flow rate of 3.1 kg/s. It then leaves the pipe as steam. The water receives 9753840 J of heating from the pipe. Calculate the...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Let M > 0 be a fixed positive definite n n matrix. A nonzero vector v 0 is called a generalized eigenvector of the n n matrix K if Kv = Mv, v 0, (8.31) where the scalar is the corresponding...
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
Predict the major products formed when: (a) Toluene is sulfonated. (b) Benzoic acid is nitrated. (c) Nitrobenzene is brominated. (d) Isopropylbenzene reacts with acetyl chloride and AlCl3. If the...
-
Use resonance theory to explain why the hydroxyl group of phenol is an activating group and an ortho-para director. Illustrate your explanation by showing the arenium ions formed when phenol reacts...
-
Phenol reacts with acetic anhydride in the presence of sodium acetate to produce the ester phenyl acetate: The CH3COOi- group of phenyl acetate, like the -OH group of phenol (Practice Problem 15.8),...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


