From models of the transition states for their reactions, predict which of the following two diastereomers of
Question:
From models of the transition states for their reactions, predict which of the following two diastereomers of 3-bromo-2-butanol should form an epoxide at the greater rate when treated with base, and explain your reasoning.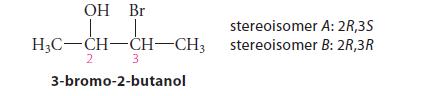
Transcribed Image Text:
OH Br H₂C-CH-CH-CH3 3 3-bromo-2-butanol 2 stereoisomer stereoisomer A: 2R,3S B: 2R,3R
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The key to solving this problem is to realize that in order for epoxide formation to occur the oxyge...View the full answer

Answered By

Antony Mutonga
I am a professional educator and writer with exceptional skills in assisting bloggers and other specializations that necessitate a fantastic writer. One of the most significant parts of being the best is that I have provided excellent service to a large number of clients. With my exceptional abilities, I have amassed a large number of references, allowing me to continue working as a respected and admired writer. As a skilled content writer, I am also a reputable IT writer with the necessary talents to turn papers into exceptional results.
4.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When the first compound shown here is treated with sodium methoxide, the only elimination product is the trans isomer. The second diastereomer (blue) gives only the cis product. Use your models and...
-
Two of the compounds given in Fig. Pl 1.78 form epoxides readily when treated with -OH, one forms an epoxid slowly, and one does not form an epoxide at all. Identify the compound(s) in each category...
-
Justin Bieber has emerged as a bona fide sports star, but in the most unlikely of sportstable tennis. At only 24 years old, Bieber has won 8 of the last 11 major table tennis tournaments over the...
-
Gems Co. uses the indirect method to prepare its statement of cash flows. The following comparative statement of financial position for 2021 and 2022 are presented: At December 31 2022 2021 Property,...
-
Information from comparative income statements and balance sheets for Microsoft and IBM is given below. (Amounts are in millions.) Use this information to answer the following questions: 1. Without...
-
Comparative income statements and balance sheets for Merck ($millions) follow: Required a. Use the following ratios to prepare a projected income statement, balance sheet, and statement of cash flows...
-
2-9. What are points of difference and why are they important?
-
Suppose that reckless driving imposes costs (in the form of medical bills) on both the drivers themselves and on pedestrians. Each mile of reckless driving costs drivers $1 and pedestrians $0.25. The...
-
Comparative data on three companies in the same service industry are given below. Required: 2. Fill in the missing information. (Round the "Turnover" and "ROI" answers to 2 decimal places.)
-
The chlorohydrin trans-2-chlorocyclohexanol reacts rapidly in base to form an epoxide. The cis stereoisomer, however, is relatively unreactive and does not give an epoxide. Explain why the two...
-
Give the product expected when each of the following alkenes is treated with MMPP. (a) trans-3-hexene (b) =CH
-
In the Challenge Solution, we could predict the change in the equilibrium price of crops but not the quantity when GM seeds are introduced. Are there any conditions on the shapes of the supply and...
-
City Feb. Cases March Cases April Cases New York 19 56 189 Los Angeles 6 12 201 Chicago 0 3 14 Houston 19 19 272 Philadelphia 0 1 5 Phoenix 23 78 289 San Antonio 6 9 95 San Diego 3 38 258 Dallas 4 13...
-
Maximize z = 2x+2y x+6y <30 4x + 2y 32 Subject to I 0 W O 0 Maximum is I = y= at
-
2. Determine the unknown force. a) Fret 3 N Right b) Fret 10. N Down F-22 N F?5N c) Feet 12 N Up F-28 N d) Fret 10. N Right F? F-12N e) Fret = 0 F-16N E-? F-12N F-15 N F-? F-30 N F-25 N F-32 N F=24N...
-
If y = ( x ^ 3 + 7 ) ^ x , compute y ' ( 1 ) .
-
To calculate activity expected duration time, the following parameter is essential? Question 7 options: Distribution time of the unit. Time associated with the failure of the unit. Optimistic or...
-
Let 1 and 2 be two nonvertical intersecting lines with slopes m1 and m2, respectively. If θ, the angle from 1 to 2, is not a right angle, then Show this using the fact that...
-
Beginning with a country that has a trade deficit, demonstrate graphically what will happen to a countrys potential output with globalization if that countrys costs of production fall. Explain your...
-
Suggest a mechanism by which ?-ionone is transformed into ?-ionone on treatment with acid. H3o -Ionone B-Ionone
-
Draw the most stable chair conformation ofdihydrocarvone CH3 -H Dihydrocarvone
-
Draw the most stable chair conformation of menthol, and label each substituent as axial orequatorial. Menthol (from peppermint oil) - CH
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


