Give the product of the following reaction, which involves an [8s + 2s] cycloaddition: + EtOC-C=C-COEt
Question:
Give the product of the following reaction, which involves an [8s + 2s] cycloaddition: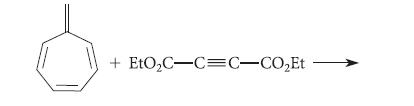
Transcribed Image Text:
+ EtO₂C-C=C-CO₂Et
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The 8s 2s cy...View the full answer

Answered By

Payal Mittal
I specialize in finance and accounts.You can ask any question related to til undergradution.Organizational behaviour and HRM are my favourites for you can always relate to them and is an art with practical knowledge base.
4.90+
226+ Reviews
778+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the product of the reaction of pentanoic acid with each of the following reagents: (a) Sodium hydroxide (b) Sodium bicarbonate (c) Thionyl chloride (d) Phosphorus tribromide (e) Benzyl alcohol,...
-
Give the product of the reaction of pentanoic acid with each of the following reagents: (a) Sodium hydroxide (b) Sodium bicarbonate (c) Thionyl chloride (d) Phosphorus tribromide (e) Benzyl alcohol,...
-
Give the product of the reaction of methyl bromide with each of the following nucleophiles: a. HO- b. -NH2 c. H2S d. HS- e. CH3O- f. CH3NH2
-
In order to have $391,185 in 26 years, how much needs to be deposited each month into a bank account whose annual rate is 1.8% with monthly compounding?
-
Ashkar Company ordered a machine on January 1, 2012, at an invoice price of $21,000. On the date of delivery, January 2, 2012, the company paid $6,000 on the machine, with the balance on credit at 10...
-
A bond has a $1,000 par value, 12 years to maturity, and an 8% annual coupon and sells for $980. a. What is its yield to maturity (YTM)? b. Assume that the yield to maturity remains constant for the...
-
* identify connections between the enquiryaction framework and the theories of change introduced earlier in the book; and
-
The following information was taken from Egeland Ltd.s adjusted trial balance as at July 31, 2016: Sales revenue....................... $2,788,800 Interest expense....................... 44,000 Cost...
-
1. Use the following information to determine food cost, food sales, and food cost percent today and to date, as well as book inventory balances for TK's Restaurant for the period October 1 5. The...
-
Show that using the HOMO from the 2-electron component and the LUMO from the 4-electron component also gives bonding overlap in a [4s + 2s] cycloaddition.
-
In the thermal ring opening of trans-3,4-dimethylcyclobutene, two products could be formed by a conrotatory mechanism, but only one is observed. Give the two possible products. Which one is observed...
-
How do the characteristics of changing environments affect uncertainty for your local coffee shop?
-
2. (40 marks) Solve for y(t) such that y" - 6y' + 15y = 2 sin(3t),
-
6. Determine output class A{ ); } public static void main(String args[]) { int x; x = 10; if (x == 10) { int y = 20; System.out.print ("x and y: y = x*2; + y); } y = 100; } System.out.print ("x and...
-
Anita and Bonita have been roommates for the past two years while they've been in graduate school. Now that they're graduating, they are each planning to move to different cities. Their one joint...
-
To what extent are business ethics assumed, or taken for granted, by people in businesses?
-
Empowered by what he has learned in this class about gender, Brady makes a friendly wager with his girfriend, Marlisa: "I bet I can guess how the men and women at the next table will behave during...
-
On June 30, the end of the first month of operations, Bastile Company prepared the following income statement, based on the absorption costing concept: If the fixed manufacturing costs were $192,000...
-
Question 6.10 Current and deferred tax worksheets and tax entries From the hip Ltd?s statement of profit or loss for the year ended 30 June 2007 and extracts from its statements of financial position...
-
Within each set, which two structures represent the same compound? A B
-
Draw a Newman projection for the most stable conformation of the compound in part (b) of Problem 2.33 that is different from the other two compounds. Draw your Newman projection about the bond...
-
Explain how you would expect the diagram of potential energy versus dihedral angle about the C2-C3 (central) carbon-carbon bond of 2,2,3,3-tetramethylbutane to differ from that for ethane (Fig. 2.3),...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


