Give the structure and stereochemistry of all products formed in each of the following reactions. Tell whetherstereoisomeric
Question:
Give the structure and stereochemistry of all products formed in each of the following reactions. Tell whether stereoisomeric products are formed in the same or different amounts.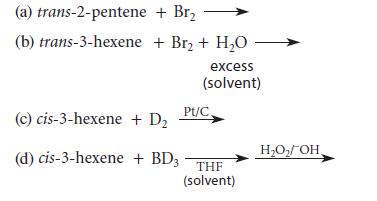
Transcribed Image Text:
(a) trans-2-pentene (b) trans-3-hexene + Br₂ + Br₂ + H₂O excess (solvent) (c) cis-3-hexene + D₂ (d) cis-3-hexene + BD3 Pt/C. THE (solvent) H₂O₂/OH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a b There are two enantiomeric and therefore chemically equivalent modes of antiaddition that give e...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure and stereochemistry of all products formed in each of the following reactions. (a) (b) trans-3-pentene Br H2O excess (solvent) ,o.roH cis-3-hexene BD3 solvent)
-
From your knowledge of the mechanism of bromine addition to alkenes, give the structure and stereochemistry of the product(s) expected in each of the following reactions. (b) Reaction of cyclopentene...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Asset allocation explains a large portion of a portfolio return. However, the implementation issues involved inthe asset allocation process may reduce the efficiency of the asset allocation strategy,...
-
Discuss the advantages and disadvantages of consumer-generated marketing. In only a few short years, consumer-generated marketing has increased exponentially. Its also known as consumer-generated...
-
Use mathematical induction to prove each of the following. a. The sum of n terms of a geometric sequence: b. x + y is a factor of x2n - y2n. a air a t ar ar+
-
What is a break-even point?
-
Making a Decision as a Financial Analyst: Preparing and Analyzing a Balance Sheet Your best friend from home writes you a letter about an investment opportunity that has come her way. A company is...
-
Refer to the bond listing table below to determine the coupon rate and maturity of a bond issued by Citigroup. Click the icon to view the bond listing from the table below. The coupon rate is % . (...
-
By answering the following questions, indicate the relationship between the two structures in each of the pairs in Fig. P7.56. Are they chair conformations of the same molecule? If so, are they...
-
Alkaline potassium permanganate (KMnO 4 ) can be used to bring about the addition of two OH groups to an alkene double bond. This reaction has been shown in several cases to be a stereospecific...
-
Visit Yahoo! Finance (finance.yahoo.com) and calculate the debt ratio of an industrial equipment manufacturer, such as Illinois Tool Works, Inc. Then calculate the debt ratio of a service-oriented...
-
The file NFL2012data.xlsx contains scores of all the NFL 2012 regular-season games. Rate the teams. Even though the Colts were 106, your ratings have the Colts as well below the average team. Can you...
-
A certain company reorders envelopes when its stock drops to 12 boxes, although demand for envelopes during lead time is normally distributed with a mean of 10 boxes and a standard deviation of 3...
-
Indicate the uses of budgeting and construct various budgets, including the cash budget, from relevant data.
-
Complete the double entry for each of the following transactions: a The owner of a business pays additional capital to the company; the cash account is debited and it is credited to the __________. b...
-
The file named Worldball.xlsx contains all the scores from the 2006 World Basketball Championships. Rate the teams. Who were the best three teams?
-
What are the most significant events in the story of how the plant survived because of its adoption of quality-based principles?
-
Would you use the adjacency matrix structure or the adjacency list structure in each of the following cases? Justify your choice. a. The graph has 10,000 vertices and 20,000 edges, and it is...
-
What is the relationship between the model and the Fischer projection?
-
How many chirality centers are present in estradiol how many stereo isomers does estradiol have?
-
Show the products, including stereo chemistry, of these SN2 reactions: a) b) c) HC Ph CH H CI + OH 2H + OCH3 CHCH CHCHCl
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


