Give the structure of the product in the reaction of each of the following esters with isotopically
Question:
Give the structure of the product in the reaction of each of the following esters with isotopically labeled sodium hydroxide, Na+ 18OH– and explain your reasoning.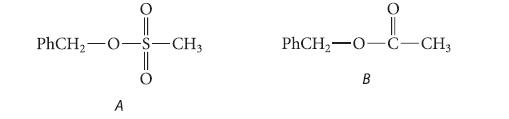
Transcribed Image Text:
PhCH₂-O- A -CH3 PhCH₂-0-C-CH3 B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Because sulfonate esters behave like alkyl halides ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of the expected organic product in the reaction of 3-phenylpropanal with each of the following: (a) Chlorine in acetic acid (b) Sodium hydroxide in ethanol, 10C (c) Sodium...
-
Give the structure of the expected organic product in the reaction of 3-phenylpropanal with each of the following: (a) Chlorine in acetic acid (b) Sodium hydroxide in ethanol, 10C (c) Sodium...
-
Give the structure of the product formed on reaction of ethyl acetoacetate with each of the following: (a) 1-Bromopentane and sodium ethoxide (b) Saponification and decarboxylation of the product in...
-
1. What options does Personal Trainer have for developing a new system? What are some specific issues and options that Susan should consider in making a decision? 2. Susan has been asked to prepare a...
-
Thelmas Amusements completed the following transactions during November 2012: Nov 1 Purchased supplies for cash, $700. 4 Purchased inventory on credit terms of 3/10, n/eom, $9,600. 8 Returned half...
-
Two 182-day, $1000000 T-bills were initially issued at a price that would yield the buyer 2.5%. If the yield required by the market remains at 2.5%, how many days before their maturity date will the...
-
In Chapter 6we came across an alternative theory as to the way in which different sources of long-term capital are included in the capital structure of a business. Can you recall what it is and...
-
Rescue Sequences, LLC, purchased inventory by issuing a $12,000, 10%, 60-day note on October 1. Prepare the journal entries for Rescue Sequences to record the purchase and payment assuming it uses a...
-
On January 1, 2020, the City of Graf pays $63,000 for a work of art to display in the local library. The city will take appropriate measures to protect and preserve the piece. However, if the work is...
-
(a) Draw the structure of the product that is formed when the following compound is heated with one equivalent of sodium methoxide in methanol. Explain your reasoning. (b) What would the product be...
-
Using an acid chloride synthesis as a first step, outline a conversion of hexanoic acid into each of the following compounds. (a) Ethyl hexanoate (b) N-methylhexanamide
-
Write a recursive method power(base, exponent) that, when called, returns base exponent For example, power(3,4) = 3 * 3 * 3 * 3. Assume that exponent is an integer greater than or equal to 1. The...
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
Demonstrate that one can also solve the initial value problem in Example 9.8 by writing the solution as a complex linear combination of the complex eigen-solutions, and then using the initial...
-
The senior management at Davis Watercraft would like to determine if it is possible to improve firm profitability by changing their existing product mix. Currently, the product mix is determined by...
-
Write a retrosynthetic analysis and then synthetic reactions that could be used to prepare racemic Meparfynol, a mild hypnotic (sleep-inducing compound), starting with compounds of four carbon atoms...
-
Write a retrosynthetic analysis and synthesis for the following transformation. OH Br HO HO
-
Synthesize the following compound using cyclopentane and ethyne (acetylene) as the sole source of carbon atoms.
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App


