Give the structures of two compounds that would give the following amine after LiAlH 4 reduction. NH
Question:
Give the structures of two compounds that would give the following amine after LiAlH4 reduction.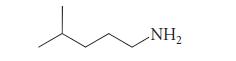
Transcribed Image Text:
NH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Among the compounds that would gi...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structures of two compounds that would give the amine (CH3)2CHCH2CH2CH2NH2 after LiAlH4 reduction.
-
Give the structures of the carbonyl compound and the amine used to form the following imines. (a) (b) (c) (d) (e) (f) NH N=CHCH, N CH
-
Give the structures of compounds A through H in the following series of reactions. HNO3 H2SO4 Zn(Hg) HCI KMnO (hot, concd.) NH2 hv NH (excess) (CHCO K NaOCH
-
Consider a 2-m-long wire of thermal diffusivity a=1 subjected to initial temperature 3x(2-x) and boundary temperature 1 and t. The governing equation is: = a at subject to T(0,t) = 1,T(2,t) =t and...
-
The accounts of Taylor Electronics Company are listed along with their balances before closing for the month ended March 31, 2012. Requirements 1. Prepare Taylor Electronics multi-step income...
-
Suppose it costs C$380 to purchase US$310.61. (a) What is the exchange rate? (b) How many U.S. dollars will you receive if you convert C$725 into U.S. dollars?
-
Briefly evaluate each of the proposed financing options from the perspective of an existing shareholder.
-
Eilers Company has two producing departments and two support departments. The following budgeted data pertain to these four departments: Required: 1. Allocate the overhead costs of the support...
-
Case: Jhon McDonald Jhon McDonald is a production manager of a company that manufactures machine parts for a heavy machinery firm. Jhon joined the company 12 years back and introduced a...
-
(a) In the catalytic hydrogenation of some nitriles to primary amines, secondary amines are obtained as by-products: Suggest a mechanism for the formation of this by-product. (b) Explain why ammonia...
-
Complete the following reactions by giving the principal organic product(s). (b) (c) Raney Ni (catalyst) heat PhCHC=N + H O II EtO-C-CH-CN LiAlH4 (excess) 1) H3O+ 2) "OH 0-C-CH3 T Ph-CH-COEt + LiAlH4...
-
There are several definitions of OD provided in this chapter. Which one do you most relate to? Why?
-
Recall from Case 1.2 that Auto Concepts is a new division of a large automobile manufacturer that has been slowly losing market share to its competitors. Auto Concepts was created to reclaim the...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a diverging lens. \((b)\) Is the image real or virtual? (c) Is it upright or...
-
Show that the ray exiting the block in Figure P33.53 is parallel to the ray entering the block. Data from Figure P33.53
-
The element is subjected to the state of stress shown. If the material is machine steel having a yield stress of \(\sigma_{Y}=750 \mathrm{MPa}\), determine the factor of safety with respect to...
-
Determine the vertical displacement of the ring at point \(B\). \(E I\) is constant. B P A
-
Suppose u(f) and both solve the linear system (a) Suppose they have the same value at any one time t1. Show that they are, in fact, the same solution: for all t. (b) What happens if for some t1 t2....
-
Assume that a trial balance is prepared with an account balance of $21,360 listed as $21,630 and an account balance of $1,500 listed as $15,000. Identify the transposition and the slide.
-
What organic products A-H would you expect from each of the following reactions? MeLi (1) NaH HELO A (2) NH4Cl,H20 OMS Ni,B (P-2), H2 NaBH MeOH (1) LAH (2) aq. H.SO4 MsCI PyT
-
Outline all steps in a synthesis that would transform 2-propanol (isopropyl alcohol) into each of the following: (a) (b) (c) (d) (e) CI
-
Which reducing agent, LiAlH4 or NaBH4, would you use to carry out the following practice problem 12.3 transformations? (a) (b) (c) (d) (e) HO HO HO OMe OMe OH
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


