Given the product distribution in Eq. 9.88, calculate the relative rate of abstraction (per hydrogen) of a
Question:
Given the product distribution in Eq. 9.88, calculate the relative rate of abstraction (per hydrogen) of a tertiary and a primary hydrogen by a chlorine atom.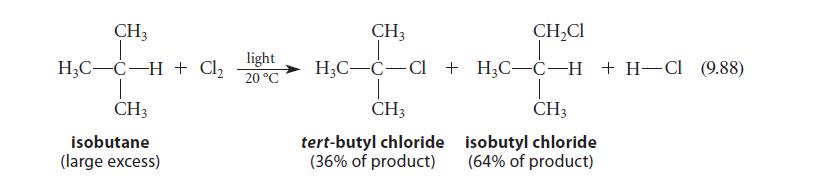
Transcribed Image Text:
CH3 H₂C-C-H + Cl₂ T CH3 isobutane (large excess) light 20 °C CH3 T CH₂Cl T H₂C-C-Cl + H₂C-C-H + H-CI (9.88) CH3 tert-butyl chloride (36% of product) T CH3 isobutyl chloride (64% of product)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
In this reaction there is only 36 of the tertiary alkyl chloride produc...View the full answer

Answered By

Michael Mulupi
I am honest,hardworking, and determined writer
4.70+
72+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The three vibrational frequencies in H 2 O (1595, 3657, and 3756 cm 1 ) are all much larger than the corresponding frequencies in D 2 O (1178, 2671, and 2788 cm 1 ). This follows from the fact that...
-
Deuterium (D) is the hydrogen isotope of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C-D...
-
Hydrogen abstraction from hydrocarbons by atomic chlorine is a mechanism for Cl · loss in the stratosphere. Consider the reaction of Cl· with ethane: C 2 H 6 (g) + Cl· (g) C 2 H...
-
A beam of light consisting of two wavelengths, 650 nm and 520 nm, is used to obtain interference fringes in a Young's double-slit experiment. Find the distance of the third bright fringe on the...
-
Jefferson County General Fund began the year 2012 with the following account balances: During 2012, Jefferson experienced the following transactions: 1. The budget was passed by the County...
-
Sophie is a single taxpayer. For the first payroll period in October 2013, she is paid wages of $3,250 monthly. Sophie claims three allowances on her Form W-4. a. Use the percentage method to...
-
3. Give the journal entry (one entry) to correct the books on January 1, 2019.
-
Manufacturing data for June and July in the Blending Department of Laurence Liquids Inc. follow: All materials are added at the start of the process. Labor and factory overhead are added evenly...
-
1. Filing status determines all except which of the following? A. The applicable standard deduction amount. b. The appropriate tax rate schedule or tax table. c. The amount used for the qualifying...
-
Explain why butane is formed as a minor by-product in the free-radical bromination of ethane.
-
Give the free-radical chain mechanism for the formation of ethyl bromide from ethane and bromine in the presence of light.
-
Identify the main users of accounting and discuss their information needs.
-
Use a substitution of the form u= ax + b to evaluate the following indefinite integral. S3x 3x+4 dx
-
Task 3 In order to support other staff to complete future risk assessments, produce a short-written report that explains. how hazards that become risks can be controlled the importance of fully...
-
let arr = [x => x + 5, x => 8, x => x * 2]; let b = X; let a = arr.reduce((acc, f) => acc + f(b), 0); If we know a is 28, what's the value of X?
-
2. (10 pts.) Identify the point symmetry elements of the structures for which the given directions are equivalent. Enumerate the elements (i.e., the individual symmetry operations) that make up the...
-
Theory Newton's second law can be written in a more general form as where is the momentum of system of N objects and is the net external force on the system. This relationship says that the rate at...
-
In Problems 1-4, estimate the error that is made by approximating the sum of the given series by the sum of the first five terms? 1. 2. 3. 4. AT+ R o0 2 (k k(k + 1) k+1
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
Give IUPAC names for the followingcompounds: (b) H;3CH-C%3CH:CH (a) CH CCH-C%3CCH CH CH (c) H H (d) CHH33H CH2C3CH CH (e) H%3D HH CH-CH (f) CHCH-H3CCH CHCH3 H
-
Draw structures corresponding to the following names: (a) 3, 3-Dimethyl-4-octyne (b) 3-Ethyl-5-methyl- 1, 6, 8-decatriyne (c) 2, 2, 5, 5-TetramethyL-3-hexyne (d) 3, 4-Dimethylcyclodecyne (e) 3,...
-
The following two hydrocarbons have been isolated from various plants in the sunflower family. Name them according to IUPAC rules. (a) CH3CH = CHC CC CCH = CHCH = CHCH = CH2 (all trails) (b) CH3C ...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


