Identify the phosphorus-containing functional groups in each of the biologically occurring compounds shown in Fig. P25.21. Choose
Question:
Identify the phosphorus-containing functional groups in each of the biologically occurring compounds shown in Fig. P25.21. Choose between phosphate monoester, phosphate diester, phosphate anhydride, and pyrophosphate monoester.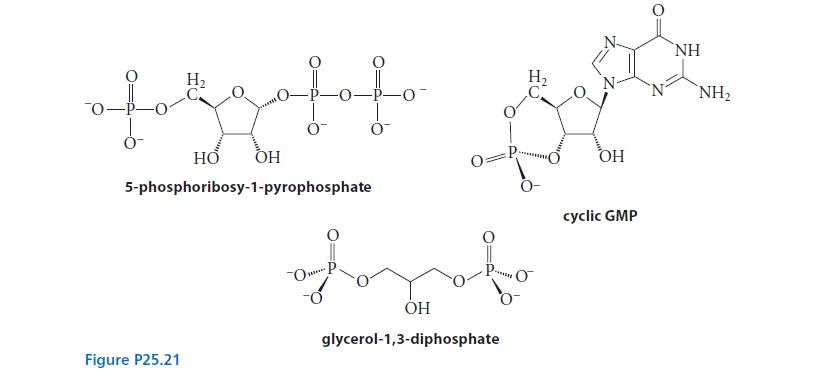
Transcribed Image Text:
of H₂ Figure P25.21 Lolo 0 HO OH 5-phosphoribosy-1-pyrophosphate 0 OP. 0₂ Dvd fort OH glycerol-1,3-diphosphate OH NH cyclic GMP NH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
phosphate monoester Ho 6 p...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify all of the functional groups in each of the following compounds: (a) (b) (c) (d) (e) (f) (g) Vitamin D3 HO OMe Aspartame O NH2 NH2 Amphetamine Me Cholesterol HO OCH2CH3 Demerol CH A...
-
Identify the functional groups in each of the following compounds: (a) H3 C--- CH2--- OH (b) (c) (d) (e) CH3CH2CH2CH2CHO H3C-N CH2CH CH2 CH3C CCH2COOH
-
Identify the functional groups in each of the following compounds: CH2CH2CH2CH2CH2CH3 Cl OH CH2CH2CH CH H2C CH3CH2CH2CH2 CH2CH2CH2CH3
-
In April 2016, Vanessa bought 100 shares in Entagon plc at a cost of 5 per share. The company went into liquidation and Vanessa received a first distribution of 40p per share in July 2020. The shares...
-
The fusion reaction between 2 H and 3 H is 3 H + 2 H 4 He + n + 17.6 MeV Using the given Q value, find the final energies of both the 4He nucleus and the neutron, assuming the initial kinetic energy...
-
Solve the given nonhomogeneous linear ODE by variation of parameters or undetermined coefficients. Show the details of your work. (D 2 + 6D + 9I)y = 16e -3x /(x 2 + 1)
-
Describe the role IT can play in supporting the project communications plan. AppendixLO1
-
The stock in Ivory Corporation is owned by Gold Corporation (80%) and Imelda (20%). Gold Corporation purchased its shares in Ivory nine years ago at a cost of $650,000, and Imelda purchased her...
-
13. Arcane Co. budgets factory overhead (FO) cost split into three activities fabrication, assembly, and inspecting. Arcane plans to manufacture four products in the following quantities: A: 1,000...
-
What would the G be for ATP hydrolysis in a cell in which the concentrations of ATP, ADP, and phosphate were all 1 mM?
-
This question refers to the biosynthesis of S-adenosylmethionine (SAM) introduced in Problem 25.15. The hydrolysis of the triphosphate leaving group in this reaction is catalyzed by the enzyme SAM...
-
Shelby wrote the check shown below to Dana. When is it payable and for howmuch? 4201 SHELBY CASE 3020 CREST DRIVE ALVIN, TX July 27, 2002 2002 Dana Locke Three Hundned Lighty Twa PAY TO THE ORDER OP...
-
Manufacturing company produces $3800 worth of products weekly. If the cost of raw materials to make this product is $400, and the labour cost is $360, calculate the productivity.
-
1-You are a very well-recognized professional in your area, with many years of experience solving international conflicts. There is a company in the middle of two European countries that are fighting...
-
Find the solution u = u(x,y) of the following problem on the set R. u du - 4, (1.4) Ju(0,y) =3y, u(x, 0) = 0. (1.5) ay
-
Scenario A Sports Club 10 Highfield Sports Club has organised a fundraising event. 300 tickets have been sold at a price of $2.50 each. Money taken at the event Percentage of money (E) taken (96)...
-
Shamrock Investments has three divisions (Green, Clover, Seamrog) organized for performance evaluation purposes as investment centers. Each division's required rate of return for purposes of...
-
(a) Show that the number of electron states in a subshell is 4 + 2. (b) By summing the number of states in each of the subshells, show that the number of states in a shell is 2n2. The sum of the...
-
The electric field due to a line charge is given by where l is a constant. Show that E is solenoidal. Show that it is also conservative. E =
-
Give the structure of a compound that meets each criterion. (a) An achiral compound C6H12O that does not give a positive Tollens test (Sec. 19.14) (b) An optically active compound C6H12O that gives a...
-
Give the structure of a compound that meets each criterion. (a) A compound C4H8O that gives a 2,4-DNP derivative but a negative haloform test (b) A compound C4H8O that gives neither a 2,4-DNP...
-
Each of the following compounds is unstable and either exists as an isomer or spontaneously decomposes to other compounds. In each case, give the more stable isomer or decomposition product and...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


