In each of the following sets, arrange the compounds in order of increasing acidity (decreasing pK a
Question:
In each of the following sets, arrange the compounds in order of increasing acidity (decreasing pKa). Explain your choices.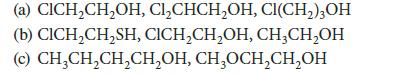
Transcribed Image Text:
(a) CICH₂CH₂OH, Cl₂CHCH₂OH, CI(CH₂)3OH (b) CICH₂CH₂SH, CICH₂CH₂OH, CH₂CH₂OH (c) CH₂CH₂CH₂CH₂OH, CH₂OCH₂CH₂OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a b c The principle is that the polar effect of a chloro substituent enhances acidity the effe...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the compounds in each of the following sets in order of decreasing pKa, highest first. Explain your reasoning. (a) CLCH2CH2SH CH3CH2OH CH3CH2SH (b) CH,CH,OH (CH3),N-CH-CH,OH (CH3)N OH
-
Arrange the compounds in each of the following groups in order of increasing solubility in water, and briefly explain your answers: a. 1-octanol; ethanol; ethyl chloride b. HOCH2(CHOH)3CH2OH;...
-
Arrange the bonds in each of the following sets in order of increasing polarity: (a) C-F, O-F, Be-F (b) O-Cl, S-Br, C-P (c) C-S, B-F, N-O
-
Five Smithtown High School students are saving up to buy their first cars. They all have after-school jobs, and their weekly salaries are listed in the table. Emily ..........................$110 Sam...
-
A successful businessman in the community has contacted the Moose County Board of Commissioners about donating income producing securities to the County to support a particular activity. Under the...
-
Anheuser-Busch InBev Companies, Inc., reported the following operating information for a recent year (in millions): Net sales ................................................$39,758 Cost of goods...
-
E 16-13 Partnership income allocationSalary allowance, bonus, and additional contributions during the year Kat and Edd formed the K & E partnership several years ago. Capital account balances on...
-
Steelcase Inc. is one of the largest manufacturers of office furniture in the United States. In Grand Rapids, Michigan, it produces filing cabinets in two departments: Fabrication and Trim Assembly....
-
You are scheduled to receive annual payments of $9,400 for each of the next 23 years. The discount rate is 7.0 percent. What is the difference in the present value if you receive these payments at...
-
Use oxidation numbers to verify that the transformation in Eq. 10.39 (also shown below) is an oxidation. HC-CH-OH ethanol O || HC-C-OH acetic acid (10.39)
-
Name the following compounds. (a) Ca(OCH 3 ) 2 (b) CuSCH 2 CH 3
-
Data on the physical inventory of Ashwood Products Company as of December 31 follow: Quantity and cost data from the last purchases invoice of the year and the next-to-the-last purchases invoice are...
-
What do you think of the gainsharing plan that Harrah's has implemented? How does an employee make more money? How much more money can they make? Is the gainsharing plan motivating employees to...
-
How do power dynamics within an organization affect employee empowerment and autonomy, and what are the best practices for creating a balanced power structure ?
-
In thinking about management and incentive structures: What recommendations do you have for the Responsible Innovation team as they seek to better embed responsible innovation within employees'...
-
How do multinational companies adapt their corporate governance procedures and decisions to accommodate the different national and regional regulatory requirements and business ethics? Requirement: I...
-
How do "complexity theory" and the concept of "emergent properties" inform our understanding of organizational dynamics, particularly in the context of nonlinear interactions and unpredictable...
-
That 1 - 1 / 2 + 1 / 3 - 1 / 4 + ... - 1 / 2( = 1 + 1 / 2 + 1 / 3 + ... + 1 / 2( - (1 + 1 / 2 + 1 / 3 + ... + 1 / n) = 1 / n + 1 + 1 / n + 2 + ... + 1 / 2n Recognize the latter expression as a...
-
Provide a draft/outline of legal research involving an indigenous Canadian woman charged with assault causing bodily harm under (Sec 267b) of the Criminal Code, where the crown wants a 12-month jail...
-
When optically active (R)-2-rnethylcyclohexanone is treated with either aqueous base or acid, racemization occurs. Explain.
-
Would you expect optically active (S)-3-methylcyclohexanonc to be race-mized on acid or base treatment in the same way as 2-methylcyclohexanonc. Explain.
-
When an optically active carboxylic acid such as (R)-2-phcnylpropanoic acid is brominated under HellVolhardZelinskii conditions, is the product optically active or racemic? Explain.
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


