In each of the following sets, arrange the three compounds in order of increasing reactivity toward base-promoted
Question:
In each of the following sets, arrange the three compounds in order of increasing reactivity toward base-promoted hydrolysis, least reactive first, and explain your reasoning.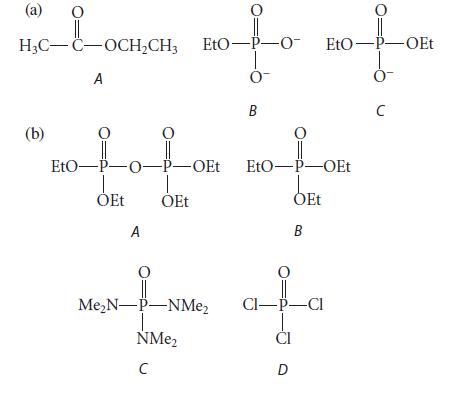
Transcribed Image Text:
(a) nofo H₂C-C-OCH₂CH3 EtO-P-O- A 0- B (b) EtO-P-O-P-OEt ỎEt A OEt O Me₂N-P-NMe₂ NMe₂ C O EtO-P-OEt 0- с EtO-P-OEt OEt B CI-P-CI CI D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a b The order ...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the compounds in each of the following sets in order of decreasing pKa, highest first. Explain your reasoning. (a) CLCH2CH2SH CH3CH2OH CH3CH2SH (b) CH,CH,OH (CH3),N-CH-CH,OH (CH3)N OH
-
Arrange the bonds in each of the following sets in order of increasing polarity: (a) C-F, O-F, Be-F (b) O-Cl, S-Br, C-P (c) C-S, B-F, N-O
-
Arrange the compounds in each of the following groups in order of increasing solubility in water, and briefly explain your answers: a. 1-octanol; ethanol; ethyl chloride b. HOCH2(CHOH)3CH2OH;...
-
Suzanne acquired the following ordinary shares in Quarine plc: She made no further acquisitions and the shares were valued at 3.20 each on 31 March 1982. On 24 July 2020, Suzanne sold 1,200 shares...
-
The counting rate from a radioactive source is 6400 counts/s. The half-life of the source is 10 s. Make a plot of the counting rate as a function of time for times up to 1 min. What is the decay...
-
Solve the initial value problem. State which rule you are using. Show each step of your calculation in detail. (D 2 - 2D)y = 6e 2x - 4e -2x , y(0) = -1, y' (0) = 6
-
What is a progress report? AppendixLO1
-
American Steel Corp. acquired the following securities in 2011: At the beginning of 2011, American Steel had a zero balance in each of its market adjustment accounts. 1. What entry or entries would...
-
The following information is available for Crane's Chocolates: (a) Calculate the direct materials price and quantity variances. Direct material price variance $ Direct material quantity variance $...
-
Complete the reactions shown in Fig. P25.24 by drawing the structures of the products, and explain your reasoning. (b) (c) afa CI H3C- + HN(CH,), (large excess) Holofo Figure P25.24 (1) TsCl OH...
-
The anomeric proton (red) in the a-anomer of glucose-1-phosphate in the proton NMR spectrum appears at 5.45. It is split into a doublet of doubletsfour lines of equal sizewith coupling constants of...
-
In 2001, the United Kingdom suffered an epidemic of foot-and-mouth disease. The graph below shows the reported number of cattle (red) and pigs (blue) that were culled each month from mid-February...
-
Listed below are the lead concentrations (in ug/g) measured in different Ayurveda medicines. Ayurveda is a traditional medical system commonly used in India. The lead concentrations listed here are...
-
The assignment states to use a movie and talk about 2 scenes where physics ideas are used. The rubric is shown and 6 big ideas that can be talked about are also attached. Background Information...
-
A series of computer and backup system failures caused the loss of most of the company records at Stotter, Incorporated. Information technology consultants for the company could recover only a few...
-
Future value of an annuity Using the values below, answer the questions that follow. (Click on the icon here in order to copy the contents of the data table below into a spreadsheet.) Deposit period...
-
Mercury, Incorporated, produces cell phones at its plant in Texas. In recent years, the company's market share has been eroded by stiff competition from overseas. Price and product quality are the...
-
What is the ratio of the wavelength of a 0.100-keV photon to the wavelength of a 0.100-keV electron?
-
To help you become familiar with the accounting standards, this case is designed to take you to the FASBs Web site and have you access various publications. Access the FASBs Web site at...
-
When the conjugate-base enolate of diethyl malonate is treated with bromobenzene, no diethyl phenylmalonate is formed. Explain why bromobenzene is inert. CH (CO,Et)2 + Br" diethyl phenylmalonate
-
The reactions of ester enolate ions are not restricted to simple alkylations. With this in mind, suggest the structure of the product formed when the enolate ion formed by the reaction of fm-butyl...
-
Outline a synthesis of each of the following compounds from a -keto ester; then show how the -keto ester itself can be prepared. CHj
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


