In either acid or base, 3-cyclohexenone comes to equilibrium with 2-cyclohexenone: (a) Explain why the equilibrium favors
Question:
In either acid or base, 3-cyclohexenone comes to equilibrium with 2-cyclohexenone: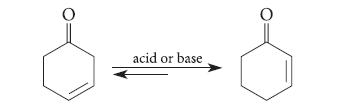
(a) Explain why the equilibrium favors the α,β-unsaturated ketone over its β,γ-unsaturated isomer.
(b) Give a mechanism for this reaction in aqueous NaOH.
(c) Give a mechanism for the same reaction in dilute aqueous H2SO4.
(d) Is the equilibrium constant for the analogous reaction of 4-methyl-3- cyclohexenone expected to be greater or smaller? Explain.
Transcribed Image Text:
acid or base O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a The equilibrium favors the aunsaturated isomer because it is conjugated Conjugation is a stabilizi...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The incorrect regarding the following structure is NH2 OH HO-P N. OH Select one O a Analogue of deoxycytidine 5-monophosphate Ob Denved from isoster replacememnt with deoxycytidine 5-monophosphate Oc...
-
The grocery retail industry across America has always tended to involve healthy competition among the various firms in it. (a) Identify the various business competitors in this industry across any...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Firms raise capital from investors by issuing shares in the primary markets. Does this imply that corporate financial managers can ignore trading of previously issued shares in the secondary market?
-
What is the basic accounting equation? Discuss.
-
Assume you are the Communications Director for a large corporation. You are leaving to pursue a better career opportunity and your successor has asked for some professional advice regarding the...
-
Recommend a strategy that management at a large pharmaceutical firm should employ to reduce the likelihood of political and legal risks that such firms face. What steps should management take to...
-
Gamblers often falsely predict the outcome of a future trial based on the outcome of previous trials. When trials are independent, the outcome of a future trial cannot be predicted based on the...
-
Orange Company Balance Sheet December 31, 20X6 and 20X5 (dollars in thousands) 20X6 20X5 Current assets: Cash and marketable securities $130 $110 Accounts receivable, net 180 180 Inventory 160 160...
-
(a) The resonance structures shown in part (a) of Fig. P22.76 can be written for an ,-unsaturated carboxylic acid. Would this type of resonance interaction increase or diminish the acidity of an ,-...
-
In 3-methyl-2-cyclohexenone the eight hydrogens H a , H b , H c , and H d can be exchanged for deuterium in CH 3 O/CH 3 OD. (a) Write curved-arrow mechanisms for the basecatalyzed exchange of...
-
Are standards like those promoted by the International Organization for Standardization (see www.iso.org) a hindrance or an opportunity for exporters?
-
As the human resource manager, how would you evaluate the training needs of your staff? How can you ensure that the training you would provide is effective? What data might be used to make your...
-
MARYLAND CORPORATION manufactures three liquid products - Alpha, Beta and Gamma using a joint process with direct materials, direct labor and overhead totaling $560,000 per batch. In addition, the...
-
Three common organizational structures. Mention one organization for each organizational structure which is following a specific organizational structure. Also, provide support to your answer by...
-
You are a retail manager at Kitchen Nightmare, a relatively new store at the mall that sells mostly items for kitchens, like forks, oven mitts, etc.. You have been open since the fall of 2021 and...
-
Examine the extent to which the Department of Veteran Affairs has established any processes or procedures to ensure knowledge retention of departing employees. Why is it important to manage the...
-
(a) Find the spectral radius of the Jacobi and Gauss-Seidel iteration matrices when (b) Is A diagonally dominant? (c) Use (10.86) to fix the optimal value of the SOR parameter. Verify that the...
-
Give codons for the following amino acids: (a) Th (b) Asp (c) Thr
-
Write a mechanism that explains the following reaction. OH 2, NaHCO3
-
Write a mechanism for the following reaction. OH H SO. H2O
-
Write a mechanism that explains formation of the products shown in the following reaction. Br, NaCI, H20o Rr CL
-
Dr. Claudia Gomez, a plastic surgeon, had just returned from a conference in which she learned of a new surgical procedure for removing wrinkles around eyes, reducing the time to perform the normal...
-
QUESTION 2 ( 2 0 Marks ) 2 . 1 REQUIRED Study the information provided below and prepare the Income Statement for the year ended 3 1 December 2 0 2 3 using the marginal costing method. INFORMATION...
-
DROP DOWN OPTIONS: FIRST SECOND THIRD FOURTH 5. Cost of new common stock A firm needs to take flotation costs into account when it is raising capital fromY True or False: The following statement...

Study smarter with the SolutionInn App


