In the Krebs cycle (tricarboxylic acid, or citric acid, cycle), the enzyme citrate synthase catalyzes the synthesis
Question:
In the Krebs cycle (tricarboxylic acid, or citric acid, cycle), the enzyme citrate synthase catalyzes the synthesis of citrate from oxaloacetate and acetyl-CoA as shown in Fig. P22.96. Assuming that acids and bases are provided as needed in the enzyme active site, propose a curvedarrow mechanism for this reaction.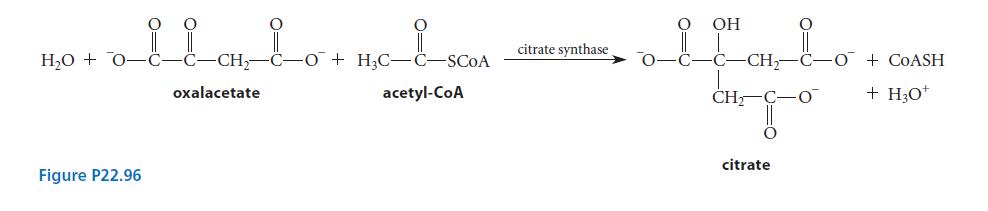
Transcribed Image Text:
H₂O + 0-C- Figure P22.96 CH₂-C-O + H₂C-C-SCOA acetyl-CoA oxalacetate citrate synthase OH oltato CH₂ CH₂- C-O + COASH + H3O+ citrate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
First form the enolate of the acetylCoA with a base in the enzyme B HO HCCSCOA HCC CSCOA HB ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Citrate synthase catalyzes the reaction The standard free energy change for the reaction is -31.5 kJ mol-1. (a) Calculate the equilibrium constant for this reaction at 37°C. (b) Would you expect...
-
Citrate synthase, one of the enzymes in the series of enzyme-catalyzed reactions known as the Krebs cycle, catalyzes the synthesis of citric acid from oxaloacetic acid and acetyl-CoA. If the...
-
The racemization of amino acids is an important reaction in a number of bacteria. This is a pyridoxal-phosphate catalyzed reaction. Outline a curved-arrow mechanism for this reaction showing clearly...
-
The stockholders' equity accounts of Marya Corporation on January 1, 2014, were as follows. Preferred Stock (9%, $100 par, cumulative, 5,000 shares authorized) ...$300,000 Common Stock ($3 stated...
-
One column total of a special journal is posted at month end to only two general ledger accounts. One of these two accounts is Accounts Receivable. What is the name of this special journal? What is...
-
What does it mean to be timely? For some, being "on time" is late, while others believe that being "on time" is actually early. Is being "on time" early or late? (Keep in mind that being on time...
-
Understand legal systems. LO.1
-
1. What is the basis for the determination that an employer should or should not be required to test applicants on an individual basis? 2. Should an employer have available as a defense that the cost...
-
Exercise 21-05 (Part Level Submission) Shamrock Leasing Company signs an agreement on January 1, 2020, to lease equipment to Cole Company. The following information relates to this agreement. 1. The...
-
Ethyl vinyl ether, EtOCH=CH 2 , hydrolyzes in weakly acidic water to acetaldehyde and ethanol. Under the same conditions, diethyl ether does not hydrolyze. Quantitative comparisons of the hydrolysis...
-
Treatment of (S)-(+)-5-methyl-2-cyclohexenone with lithium dimethylcuprate gives, after protonolysis, a good yield of a mixture containing mostly a dextrorotatory ketone A and a trace of an optically...
-
Write a program that displays a clock and sets the time with the input from three text fields, as shown in Figure 16.38b. Use the ClockPane in Listing 14.21. Resize the clock to the center of the...
-
Encouraging you to sit back and watch a full hour of one of your favorite shows on prime-time television. However, instead of getting up during the commercial break or fast forwarding through the...
-
A family member has been recently diagnosed with a heart condition that requires replacing a heart valve. She points out that if she goes to India, the surgery cost is about 60% cheaper on average...
-
Based on the case of Bowers Machine Parts. Critically analyze why people were not doing their best and critically explain why hiring a consultant might solve the issue. Justify your answer by using...
-
Identify a few strategies for sustainability effectiveness. Should sustainability be a corporation's top priority? Why or why not? What are the challenges associated with implementing sustainable...
-
Answer the following questions for the topic you want to write about. Type your answers in a separate Word document. What is the issue or debatable idea you might write about? What is debatable about...
-
Use the conjugate gradient method to solve the system in Exercise 10.5.31. How many iterations do you need to obtain the solution that is accurate to 2 decimal places? How does this compare to the...
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
Treating a hindered alkene such as 2-methyl-2-butene with BH3: THF leads to the for- mation of a dialkylborane instead of a trialkylborane. When 2 mol of 2-methyl-2-butene is added to 1 mol of BH3,...
-
Specify the appropriate alkene and reagents for synthesis of each of the following alcohols by hydroboration-oxidation. (a) (b) (c) (d) (e) (f) CH 2 OH
-
Starting with any needed alkene (or cycloalkene) and assuming you have deuterioacetic acid (CH3CO2D) available, outline syntheses of the following deuterium-labeled compounds. (a) (CH3)2CHCH2CH2D (b)...
-
Construction of consumer price index number for the given goods and services. Item Weight in % Base period price Current period price Food 35 150 145 Fuel 10 25 23 Cloth 20 75 65 Rent 15 30 30 Misc....
-
Gammaro Corporation has found that 80% of its sales in any given month are credit sales, while the remainder are cash sales of the credit sales, Gammaro Corporation has experienced the following...
-
Swifty Company estimates that 2022 sales will be $43,200 in quarter 1,$51,840 in quarter 2 , and $62,640 in quarter 3 , Cost of goods sold is 50% of sales. Management desires to have ending...

Study smarter with the SolutionInn App


