In the structure of acetyl-CoA (Fig. 25.1), point out and identify the phosphorus-containing functional groups. HC-C- HC-C-SCOA
Question:
In the structure of acetyl-CoA (Fig. 25.1), point out and identify the phosphorus-containing functional groups.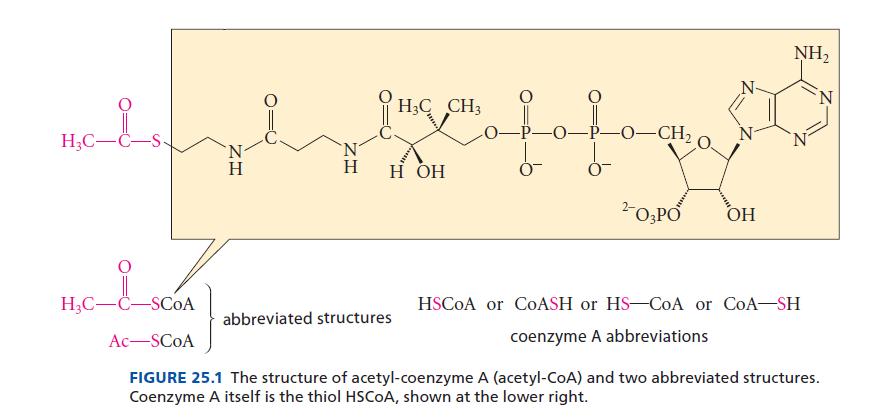
Transcribed Image Text:
H₂C-C- H₂C-C-SCOA Ac-SCOA ZH Η H3C CH3 slave fofagart —O–CH, H H OH 2- O3PO OH abbreviated structures NH₂ HSCOA or COASH or HS-CoA or CoA-SH coenzyme A abbreviations N FIGURE 25.1 The structure of acetyl-coenzyme A (acetyl-CoA) and two abbreviated structures. Coenzyme A itself is the thiol HSCOA, shown at the lower right.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
HgC thioester HC ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the etlryl groups and the methyl groups in the structure of 4-sec-butyl-5ethyl-3- methyloctane, the compound discussed in Study problem 2.5. Note that these groups are not necessarily...
-
In a structure containing a pentacoordinate phosphorus atom, the bonds to three of the groups bound to phosphorus (called equatorial groups) lie in a plane containing the phosphorus atom (shaded in...
-
In the structure of 4- isopropy 1-2,4,5-trimethylheptane (Problem 2.9) (a) Identify the primary, secondary, tertiary, and quaternary carbons. (b) Identify the primary, secondary, and tertiary...
-
Three brothers, Daniel, David and Derrick have been discussing their respective taxation affairs and how much they dislike paying tax. None of them are Scottish taxpayers. Daniel's income for tax...
-
A 1.00-mg sample of substance of atomic mass 59.934 u emits particles with an activity of 1.131 Ci. Find the decay constant for this substance in s 1 and its half-life in years.
-
Do the situations in FIGURE Q31.6 represent possible electromagnetic waves? If not, why not? a. b. 3000 V/m Vem 10 y
-
The number of four-letter passwords that can be created when no letter can be repeated USING AND INTERPRETING CONCEPTS
-
At December 31, 2013, Obermeyer Imports reported the following information on its balance sheet. Accounts receivable $250,000 Less: Allowance for doubtful accounts 15,000 During 2014, the company had...
-
Can you please help me with this question? In January 2022, the management of Clinton Corporation, a publicly-traded company, decides that it has sufficient cash to purchase some debt and equity...
-
Given the pK a values of methyl phosphate shown in this section, calculate the percentage of the un-ionized form, the monoanion form, and the di-anion form at pH 7.4.
-
Draw a structure for each of the following thioesters: (a) Cyclohexyl thiobenzoate (b) S-isopropyl butanethioate (c) S-phenyl cyclohexanecarbothioate
-
Revenue Recognition Nimble Health and Racquet Club (NHRC), which operates eight clubs in the Chicago metropolitan area, offers one-year memberships the members may use any of the eight facilities but...
-
Explain the principles of database normalization and denormalization, delineating their respective roles in optimizing data storage efficiency, query performance, and data integrity in relational...
-
Asymptotic Computational Complexity O(): Calculate the time complexity of each function below and explain your reasoning. Write your answers on paper and submit a scanned copy. (5 pts each) def...
-
Happy Valley Software has developed a new meteorology software package that will likely revolutionize the weather forecasting industry. They are looking to market the software to the following three...
-
Please read the essay Nasty Women Have Much Work To Do from Alexandra Petri on pages 45-47. In your discussion post, please share your thoughts on what specific strategies she uses to create tone and...
-
We live in an increasingly hyper-competitive global marketplace, where firms are fighting to stay lean and flexible in an effort to satisfy increasingly diverse and specialized consumer demand. In...
-
An electron is confined in a one-dimensional box of length L. Another electron is confined in a box of length 2 L. Both are in the ground state. What is the ratio of their energies E2L/ EL?
-
A crop-dusting plane flies over a level field at a height of 25 ft. If the dust leaves the plane through a 30 angle and hits the ground after the plane travels 75 ft, how wide a strip is dusted? See...
-
Explain how you would distinguish the compounds within each set by a simple chemical test with readily observable results, such as solubility in acid or base, evolution of a gas, and so forth....
-
(a) Give the structure of cocaine (Fig. 23.4) as it would exist in 1 M aqueous HCI solution. (b) What products would form if cocaine were treated with an excess of aqueous NaOH and heat? (c) What...
-
How would the basicity of trifluralin, a widely used herbicide, compare with that of N,N-diethylaniline: much greater, about the same, or much less? Explain Et Et O,N NO CF trifluralin
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


