Phosphoric acid, H 3 PO 4 , has the following structure. (a) Draw the structure of trimethyl
Question:
Phosphoric acid, H3PO4, has the following structure.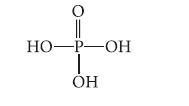
(a) Draw the structure of trimethyl phosphate.
(b) Draw the structure of the monoethyl ester of phosphoric acid.
Transcribed Image Text:
0 HO-P-OН OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a b on hoonoor ho ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Phosphoric acid (H3PO4) is an important industrial chemical used in fertilizers, in detergents, and in the food industry. It is produced by two different methods. In the electric furnace method,...
-
An organic acid has pK a = 8.95. What is its K a value? Where does the acid fit in Table 16.2? Data given in Table 16.2 Increasing Acid Strength TABLE 16.2 Acid Name Perchloric acid Sulfuric acid...
-
Epinephrine hydrochloride has a pK a value of 9.53. What is the value of K a ? Where does the acid fit in Table 16.2? Data given in Table 16.2 Increasing Acid Strength TABLE 16.2 Acid Name Perchloric...
-
Currently the rate of return of the risk-free government bond is 3% while the expected rate of return on the market is 11%. Using CAPM, calculate the rate of return required for A plc who has a beta...
-
Palmer Company had an Accounts Receivable balance of $85,000 and an Allowance for Bad Debts balance of $3,400 (credit) at the end of the year (before any adjusting entry). Credit sales for the year...
-
A propeller is rotating about an axis perpendicular to its center, as the drawing shows. The axis is parallel to the ground. An arrow is fired at the propeller, travels parallel to the axis, and...
-
Another trade-off PMs have to make is between team process and progressthe purpose being to LO6 keep the peace, give the team an occasional rest, protect the larger organization or other projects,...
-
Cybernetronics Inc. (Cyber) is a Canadian-owned public company which designs and manufactures communications and control systems. The company's year end is May 31. It is now June 2018. You, CPA, are...
-
Based on the following data, what is the accounts receivable turnover? Sales on account during year $524,466 Cost of goods sold during year 179,738 Accounts receivable, beginning of year 41,692...
-
Draw both the complete structure and the abbreviated structure, and give another name for each of the following compounds. (a) Isopropyl methanesulfonate (b) Methyl p-toluenesulfonate (c) Phenyl...
-
Tell whether each of the following compounds can be prepared by the reaction of a Grignard reagent with ethylene oxide. If so, show the reaction; if not, explain why and give a different synthesis...
-
A certain hydrocarbon had the molecular formula C16H26 and contained two triple bonds. Ozonolysis gave CH3(CH2)4CO2H and HO2CCH2CH2CO2H as the only products. Suggest a reasonable structure for this...
-
Admin Support Cereal Bars Square Foot 1,250 1,500 7,500 7,000 # of employees 14 11 42 59 # of machine batches 0 0 14 27 # of computers 17 21 35 30 Costs 32,000.32 21,740.21 The Support department...
-
Compare and contrast the differences between innovation and creativity. Does one lead to the other? If so, please explain. Why is innovation important? Who within the organization is responsible for...
-
Using the tables from Check your Consumer Surplus and Producer Surplus activities, find the equilibrium price and quantity in the market for cheese-stuffed jalapeno peppers. What is the total surplus...
-
We decided to use Gehan's two-stage design for this purpose. In the first stage, we will discard the new treatment if no patient out of n0 patients. Suppose the probability we can tolerate to discard...
-
Claude Haridge was involved in a demonstration. He threw a paint balloon at a bus and some of the paint flecks hits a nearby officer, so Haridge was transported to police cells. At the cells Special...
-
Sketch the graph of f(x) = (x - 2)2 - 4 using translations. Discuss.
-
What is the mode?
-
One of the steps in the pentose phosphate pathway for glucose catabolism is the reaction of xylulose 5-phosphate with ribose 5-phosphate in the presence of a transketolase to give glyceraldehydes...
-
The amino acid tyrosine is biologically degraded by a series of steps that include the following transformations: The double-bond isomerization of maleoylacetoacetate to fumaroyl acetoacetate is...
-
Propose a mechanism for the conversion of fumaroyl acetoacetate to fumarate plus acetoacetate (Problem 29.49).
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


