Potassium tri(isopropoxy)borohydride sometimes finds use as a source of nucleophilic H: (hydride ion). Suggest a mechanism for
Question:
Potassium tri(isopropoxy)borohydride sometimes finds use as a source of nucleophilic H:– (hydride ion).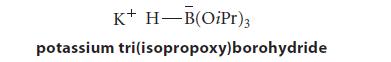
Suggest a mechanism for the substitution reaction in Fig. P18.91 that accounts for the stereochemistry of the reaction.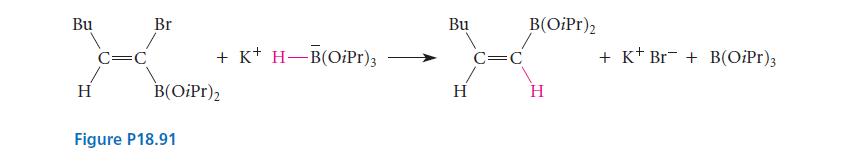
Transcribed Image Text:
K+ H-B(OiPr)3 potassium tri(isopropoxy)borohydride
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The hydride of potassium triisopropoxyborohydride forms a Lewis acidbase associ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
We have seen that NaH is a strong base but a weak nucleophile. In contrast, lithium aluminum hydride (LAH) is a reagent that can serve as a source of nucleophilic hydride ion: In this case, LAH...
-
The reaction between potassium superoxide, KO2, and CO2, 4 KO2 + 2 CO2 2K2CO3 + 3 O2 is used as a source of O2 and absorber of CO2 in self-contained breathing equipment used by rescue workers. (a)...
-
The rate law for the substitution reaction of 2-bromobutane and HO- in 75% ethanol and 25% water at 30C is rate = 3.20 10-5[2-bromobutane][HO-] + 1.5 10-6[2-bromobutane] What percentage of the...
-
The Holtz Corporation acquired 80 percent of the 100,000 outstanding voting shares of Devine, Inc., for $7.20 per share on January 1, 2014. The remaining 20 percent of Devines shares also traded...
-
The adjusted trial balances of Superior International, Inc., at August 31, 2012, and August 31, 2011, include the following amounts: Analysis of the accounts at August 31, 2012, reveals the following...
-
Harrigan Service Company, Inc., was incorporated by Ian Harrigan and five other managers. The following activities occurred during the year: a. Received $60,000 cash from the managers; each was...
-
After purchasing one of these products, why might you experience postpurchase cognitive dissonance? What might the seller do to help you resolve this dissonance? BATTLE OF THE TITANS: AMAZON ECHO VS....
-
Siberian Ski Company recently expanded its manufacturing capacity, which will allow it to produce up to 15,000 pairs of cross-country skis of the mountaineering model or the touring model. The sales...
-
hen Alyssa Weinstein died, she left her estate to her spouse and daughter as shown below. Her assets had the following tax costs and fair market values (FMV): Asset Beneficiary Jewelry - Cost =...
-
Citalopram is used as an antidepressant. A biologist, Heywood U. Clonum, has argued that this compound could be hazardous because it could release toxic cyanide ( C N) and fluoride ions by...
-
Bombykol is the mating pheromone of the female silkworm moth. Using the result in Problem 18.89, propose a synthesis of bombykol, using both HC C(CH 2 ) 9 OH (10-undecyne-1-ol) and 1-pentyne as...
-
Consider Exercise 15.3 once again. Three-factor interactions are often not significant and, even if they are, they are difficult to interpret. The interaction ABD appears to be important. To gain...
-
Ginger Tyler comes into Johns Medical Center for her routine office visit. Her co-payment is $50.00. She hands the office manager $60.00. The $10.00 change should be taken from which cash management?...
-
Do you believe that the labour laws that are currently in place (i.e., the Ontario Labour Relations Act) are sufficient to guarantee workers have adequate voice and equity in the workplace? Explain...
-
The DSV Partnership decided to liquidate as of June 30, 20X5. Its balance sheet as of this date follows: Assets Cash Accounts Receivable (net) Inventories DSV PARTNERSHIP Balance Sheet At June 30,...
-
Below what IQ does .27 of the population fall if the mean is 100 with a standard deviation of 15? (Don't round off IQ score.)
-
1. Can modern day roles be placed in the paradigm of masters, overseers, drivers, and slaves? If so, describe a parallel to these relationships you could interpret through this type of lens. If not,...
-
Find the eigenvalues and a basis for the each of the eigenspaces of the following matrices. Which are complete? 6 0000 2121 846 164 0011 1010 102- , 020 131 0110 0 131 2 644 255 1011 1010 41 34
-
DC has unused FTC carryover from 2017 in the separate category for GC income as the result of income generated by a foreign branch. The income was foreign source general category income. In 2018 the...
-
In contrast to the reaction with dilute alkali (Section 18.6), when concentrated solutions of NaOH are used, acetoacetic esters undergo cleavage as shown below.
-
(a) What product would you expect from a Dieckmann condensation of diethyl heptanedioate? (b) Can you account for the fact that diethyl pentanedioate (diethyl glutarate) does not undergo a Dieckmann...
-
Write a detailed mechanism for the following reaction. OEt NaOEt EtO OEt OEtEtO Ethyl crotonate Diethyl oxalate
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


