Propose a sequence of reactions for carrying out the following conversion. Br- C-CH3 HOCH,CH C-CH3 (19.57)
Question:
Propose a sequence of reactions for carrying out the following conversion.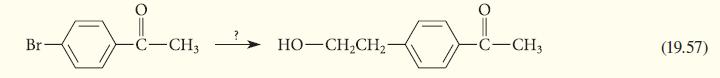
Transcribed Image Text:
Br- C-CH3 HO–CH,CH C-CH3 (19.57)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
It might seem that the way to effect this conversion would be to convert the starting halide into th...View the full answer

Answered By

John Aketch
I am a dedicated person with high degree of professionalism, particularly in academic writing. My desire is to is to make students excel in their academic endeavor.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suggest a sequence of reactions for carrying out each of the following conversions. (a) Butyric acid to 3-methyl-3-hexanol (b) Propionic acid to 3-pentanone
-
Suggest a suitable series of reactions for carrying out each of the following synthetic transformations: CH CH32 CO-H to SO3H CH3 CO H CH to (CH3)3 CH C to 0 OCH OCH O-N to OCH C(CH)s
-
Outline a sequence of reactions for the conversion of 3-pentanol into 3-bromopentane.
-
What is the difference between an optimistic approach and a pessimistic approach to decision making under assumed uncertainty?
-
The accountant for Reva Stewart, CPA, P.C., has posted adjusting entries (a) through (e) to the accounts at December 31, 2012. Selected balance sheet accounts and all the revenues and expenses of the...
-
1. Mullinix Inc. reported the following information: net income, $55,000; decrease in accounts receivable, $12,000; decrease in accounts payable, $6,500; and depreciation expense, $10,000. What...
-
Identify the four determinant attributes that set apart the Amazon, Google, and Apple smart home devices. Use those attributes to develop a compensatory purchasing model similar to the one in Exhibit...
-
Dividend yield is the annual dividend paid by a company expressed as a percentage of the price of the stock (Dividend/Stock Price 3 100). The dividend yield for the Dow Jones Industrial Average...
-
The post-closing trial balances of two proprietorships on January 1, 2018, are presented below. Santos Company Luigy Company Debit Credit Debit Credit Cash $28,000 $24,000 Accounts receivable 35,000...
-
Deduce the structures of the following compounds. (a) CHgO: IR 1720, 2710 cm- NMR in Fig. 19.4
-
Provide a substitutive name for the following compound. (The numbers are used in the solution that follows.) OH wp-fm- 2 5 HC-C-CH-CH-CH-CH-CH-CH HC CH
-
At what time of day are you sleepiest? At what time of day are you least sleepy? Sleepiness has two cycles, a circadian rhythm with a period of approximately 24 hours and an ultradian rhythm with a...
-
Why is it critical to immediately contact your Engagement Partner when you suspect or identify non-compliance? He or she will ensure that the non-compliance doesn't affect the Client's reputation He...
-
Question 9: Determine the current and its direction, in each resistor, for the circuit shown below. Show your calculations. R=152 9.0 V + 12V ww R=75 2 R3= 50
-
how can The High - Tech Way To Recycle Clothes sustainable. and what they offer and what are their ecofriendly
-
James Bondbuyer purchases a Treasury bond on Monday, May 2, regular way settlement. The bond pays interest on January 15 and July 15. How many days of accrued interest will be owed to the seller? A...
-
Aviation and air traffic control have come a long way in the last 100-years. Some believethat we have reached a plateau and that growth in aviation will stop. Aviation may go the way of the railroads...
-
A matrix A has eigenvalues -1 and 2 and associated eigenvectors Write down the matrix form of the linear transformation L[u] = A u in terms of (a) The standard basis e1, e2; (b) The basis consisting...
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
When acrolein (propenal) reacts with hydrazine, the product is a dihydropyrazole: Suggest a mechanism that explains this reaction. H + H2N-NH2 Acrolein Hydrazine A dihydropyrazole
-
(a) Propose step-by-step mechanisms for both transformations of the Robinson annulations sequence just shown. (b) Would you expect 2-methylcyclohexane-1, 3-dione to be more or less acidic than...
-
Outline reasonable mechanisms that account for the products of the following Mannich reactions: (a) (b) (c) NMe2 Me2N NMe2 + 2 Me2NH CH3 CH
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


