Represent each of the following compounds with a skeletal structure. (a) CH3 I CHCH,CH,CHCHC(CH3)3 T CH3 (b)
Question:
Represent each of the following compounds with a skeletal structure.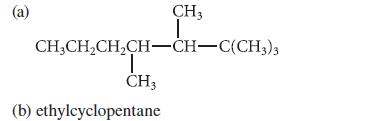
Transcribed Image Text:
(a) CH3 I CHỊCH,CH,CH–CH–C(CH3)3 T CH3 (b) ethylcyclopentane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a 2234te...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Redraw the structures in Problem 2.14 using abbreviations for substituent groups. For structure (a), draw one structure that shows as many abbreviated methyl groups as possible. Problem 2.14...
-
Discuss the potential disadvantages of brainstorming? How can you overcome the challenges?
-
Determine the structure of each of the following compounds based on its mass, IR, and 1H NMR spectra. a. b. 100 u 50 27 114 20 40 60 80 100 120 m/z 3 2 2 28 2.9 10 13 14 15 6 6 5 2 (ppm) frequency...
-
Suppose that you borrow $1000.00 from a friend and promise to pay back $1975.00 in 5 years. What simple interest rate will you pay?
-
Investigative Report: Ensuring Fair Employment Practices Abroad Nikes image took a big hit a few years ago, when the company became associated with sweatshop conditions in Asian factories that...
-
The steel plane truss shown in the figure is loaded by three forces P, each of which is 490 kN. The truss members each have a cross-sectional area of 3900 mm2 and are connected by pins each with a...
-
Greeks on campus. The question-and-answer column of a campus newspaper was asked what percentage of the campus was Greek (that is, members of fraternities or sororities). The answer given was that...
-
Are Brand Extensions Good or Bad? Some critics vigorously denounce the practice of brand extensions, because they feel that too often companies lose focus and consumers become confused. Other experts...
-
Each year, Worrix Corporation manufactures and sells 3,400 premium-quality multimedia projectors at $12,400 per unit. At the current production level, the firm's manufacturing costs include variable...
-
Draw a structure for (CH 3 CH 2 CH 2 ) 2 CHCH(CH 2 CH 3 ) 2 in which all carboncarbon bonds are shown explicitly; then name the compound.
-
Draw structures for all isomers of (a) Hexane and (b) Heptane. Give their systematic names.
-
A solid sphere is rolling on a surface. What fraction of its total kinetic energy is in the form of rotational kinetic energy about the center of mass?
-
If f ( x ) = ( 1 3 - In ( x ) ) ^ 8 , determine f ' ( 1 ) .
-
1. ThestocksAandBhavethefollowingdistributionsofreturns. A B Probability State1 3 4 0.2 State2 5 2 0.3 State3 4 8 0.2 State4 6 5 0.1 State5 6 1 0.2 2....
-
Define nested designs. Explain why the nested designs are important.
-
3 x y 3 + x y = l n ( x ) solve for d y d x
-
Let ln ( xy ) + y ^ 8 = x ^ 7 + 2 . Find dy / dx .
-
List the steps of the action of most nonsteroid hormones.
-
How has the too-big-to-fail policy been limited in the FDICIA legislation? How might limiting the too-big-to-fail policy help reduce the risk of a future banking crisis?
-
Explain which of these reactions would have the faster rate: CI CH3 CI CH,CH,CH, + CH;-N: CH3 or CH;-NH2 CH,CH,CH, +
-
Heating ethanol with sulfuric acid is one method used for the preparation of diethyl ether. Show all of the steps in the mechanism for this reaction: H,SO4 2 CH;CH,OH CH;CH,-0-CH,CH, + H2O
-
When an aqueous solution of (R)-2-butanol is treated with a catalytic amount of sulfuric acid, slow racemization of the alcohol occurs. Show all of the steps in the mechanism for this process.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


