The hydrolysis of acetyl chloride is 7800 times faster than the hydrolysis of acetyl fluoride. Which factor
Question:
The hydrolysis of acetyl chloride is 7800 times faster than the hydrolysis of acetyl fluoride.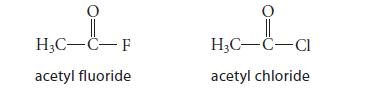
Which factor is more important in determining the relative hydrolysis rate: resonance stabilization of the carbonyl compound or polar stabilization of the transition state? Explain how you know.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





