The molecules nitromethane and 2-propanol have roughly the same shape and molecular mass. Liquid 2-propanol contains hydrogen
Question:
The molecules nitromethane and 2-propanol have roughly the same shape and molecular mass.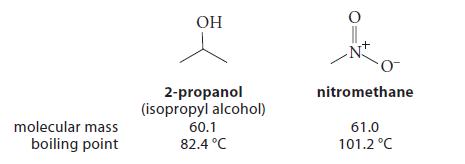
Liquid 2-propanol contains hydrogen bonds, but liquid nitromethane does not. Yet nitromethane has the higher boiling point. Why does nitromethane have such a high boiling point? What physical properties of the two molecules could you look up to support your answer?
Transcribed Image Text:
molecular mass boiling point OH 2-propanol (isopropyl alcohol) 60.1 82.4 °C nitromethane 61.0 101.2 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
nitromethane and 2propanol have similar molecular mass but different boiling points Nitromethane has ...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Discussion 1- One key component of descriptive writing is showing the reader something rather than telling the reader. For example, I could tell you that I was cold. Or, I could show you: I shivered,...
-
In order to evaluate lim f(a+h)-f(), it is necessary to evaluate f(a + h). h xa For f(x) = x 3, f(a+h) =
-
Was there sufficient evidence to prove that Reyes violated the AECA and related regulations?
-
A simple beam AB supports two connected wheel loads P and 2P that are distance d apart (see figure). The wheels may be placed at any distance x from the left-hand support of the beam. (a) Determine...
-
What is the corporate culture in your firm?
-
Explain the difference between a private-purpose trust and a public-purpose trust. How does the reporting for the two types of trusts differ?
-
= Homework: Week Three Question 9, E11-13 (simil... Part 1 of 4 HW Score: 0%, 0 of 22 points Score: 0 of 1 Save Kramer Koal Company, Inc. purchased a new mining machine at a total cost of $1,800,000...
-
(a) One of the following compounds is an unusual example of a salt that is soluble in hydrocarbon solvents. Which one is it? Explain your choice. (b) Which of the following would be present in...
-
Without consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) 1-hexanol, 2-pentanol, tert-butyl alcohol (b)...
-
The accountant for Cyclone Construction, Inc., posted adjusting entries (a) through (e) to the accounts at July 31, 2012. Selected balance sheet accounts and all the revenues and expenses of the...
-
As a new principal, I assigned a teacher to a different grade for the coming year. I did not expect to cause the anxiety it did. The teacher first came to me in tears and begged for her assignment to...
-
Peruse the following websites to learn about the different ways of categorizing leadership. 1. https://www.businessnewsdaily.com/9789-leadership-types.html 2....
-
Making Consumer Choices The Espresso Machine (25 points) In real life, you must often make choices about whether to buy something pre-made or make it yourself. There are many things to consider:...
-
1) Read over the article/case and summarize what it is referring to in your own words. 2) What type of leadership traits can you describe in the case study? Use materials both from the handout and...
-
After reading or watching, https://smallbusiness.chron.com/internal-analysis-important-80513.html https://www.indeed.com/career-advice/career-development/internal-analysis...
-
Distinguish between normal and paradoxical sleep.
-
Define cultural intelligence. Cite the books or journal articles you found in Capella's library. Explain why cultural intelligence is important for HR practitioners and other organizational managers.
-
Identify the most acidic site in thesecompounds: NH2 CH.COCH,CH,CH3 c) a) b) CH3 e) CH;CH,CH,COH d) CH,CH,CCH,CH3
-
Suggest explanations for the origins of "ibu," "pro," and "fen" in the name ibuprofen. Provide a systematic name for thiscompound OH O,N. NO2 NO2 Picric acid
-
The pK a for the picric acid is 0.42. Explain why it is such a strong acid.
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


