The optically active alkyl halide in Eq. 9.61 reacts at 60 C in anhydrous methanol solvent to
Question:
The optically active alkyl halide in Eq. 9.61 reacts at 60 °C in anhydrous methanol solvent to give a methyl ether A plus alkenes.
The substitution reaction is reported to occur with 66% racemization and 34% inversion. Give the structure of ether A and state how much of each enantiomer of A is formed.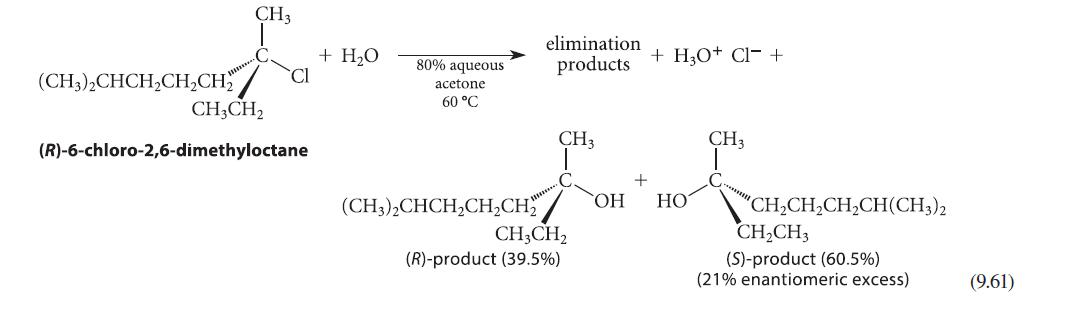
Transcribed Image Text:
(CH3)2CHCH₂CH₂CH₂ CH3 CH3CH₂ Cl (R)-6-chloro-2,6-dimethyloctane + H₂O 80% aqueous acetone 60 °C elimination products CH3 C (CH3)2CHCH₂CH₂CH₂OH CH3CH₂ (R)-product (39.5%) + + H₂O+ Cl- + HO CH3 "CH,CH,CH,CH(CH3)2 CH₂CH3 (S)-product (60.5%) (21% enantiomeric excess) (9.61)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The amount of racemization 66 corresponds to 33 of the S product ...View the full answer

Answered By

Nicole omwa
Being a highly skilled tutor with at least 5 years of tutoring experience in different areas, I learned how to help diverse learners in writing drafts of research papers, actual research papers and locate credible sources. My assurance is built upon my varied knowledge of a variety of subjects. Furthermore, my involvement and interaction with numerous learners of all levels has allowed me to understand my clients' specific demands. Ultimately, this has aided me in being a better coach to learners to better their grades. Essentially, my responsibilities as a tutor would include:
Teaching abilities that assist pupils in enhancing their academic performance
Personal interaction with learners to make them understand abstract concepts
Inducing new skills and knowledge into their academic journeys
Fostering individual reflection, and independent and critical thinking
Editing and proofreading
Because I am constantly available to respond to your queries, you may decide to rely on me whenever you require my assistance. As an assurance, my knowledge skills and expertise enable me to quickly assist learners with different academic challenges in areas with difficulty in understanding. Ultimately, I believe that I am a reliable tutor concerned about my learner's needs and interests to solve their urgent projects. My purpose is always to assist them in comprehending abstract schoolwork and mastering their subjects. I also understand that plagiarism is a severe offense and has serious ramifications. Owing to this, I always make it a point to educate learners on the numerous strategies to have uniquely unique solutions. I am familiar with the following formatting styles:
MLA
APA
Harvard
Chicago
IEEE
Communication is always the key in every interaction with my learners. Hence, I provide timely communication about the progress of assigned projects. As a result, I make sure that I maintain excellent communication with all of my clients. I can engage with all of my customers more effectively, assisting them with their unique academic demands. Furthermore, I attempt to establish a solid working relationship with my leaners I have exceptional abilities in the below areas;
Sociology
History
Nursing
Psychology
Literature
Health and Medicine
Chemistry
Biology
Management
Marketing
Business
Earth Science
Environmental Studies
Education
Being a teacher who aces in diverse fields, I provide various academic tasks, which include;
Academic Reports
Movie Reviews
Literature Reviews
Annotated bibliographies
Lab reports
Discussion posts
Dissertations
Case study analyses
Research proposals
Argumentative Essays
I guarantee you high-quality Papers!!!!!
5.00+
17+ Reviews
32+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Give the mechanistic symbols (SN1, SN2, E1, E2) that are most consistent with each of the following statements: (a) Methyl halides react with sodium ethoxide in ethanol only by this mechanism. (b)...
-
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: At equilibrium the total pressure in the container is 1.300 atm. Find the values of Kp...
-
The following information was abstracted from the accounts of the General Fund of the City of Rome after the books had been closed for the fiscal year ended June 30, 2012. During the year, purchase...
-
Sir Corporation is a 90 percent-owned subsidiary of Pit Corporation, acquired several years ago at book value equal to fair value. For 2011 and 2012, Pit and Sir report the following: 2011 2012 Pit's...
-
P 16-10 Recording new partner investmentVarious situations The AT Partnership was organized several years ago, and on January 1, 2016, the partners agree to admit Car for a 40 percent interest in...
-
You are bearish on Telecom and decide to sell short 100 shares at the current market price of $50 per share. a. How much in cash or securities must you put into your brokerage account if the brokers...
-
Built-Tight is preparing its master budget for the quarter ended September 30, 2017. Budgeted sales and cash payments for product costs for the quarter follow: July August September Budgeted sales $...
-
Give the products of the following reactions. Show the curved-arrow notation for each. (a) HC-Li + CHOH (b) (CH,),CHCH, MgCl + H,O
-
Write a curved-arrow mechanism for formation of the rearrangement product shown in Eq. 9.60. CH3 CH3 I T H3C-C- CH 3 -CH-Cl EtOH 80 C CH3 CH3 | I H3C-C- | OEt + -CH-CH3 EtOH Cl + other products...
-
Claire Corporation is planning to issue bonds with a face value of $100,000 and a coupon rate of 8 percent. The bonds mature in two years and pay interest quarterly every March 31, June 30, September...
-
Find the unknown angle measures. 49 60 Drawing is not to scale. I = y = In S
-
Q5 For this question, use data from only restaurants with between 50 and 60 items in the data set. Predict total fat from cholesterol, total carbs, vitamin a, and restaurant. Remove any...
-
A meteorologist believes that there is a relationship between the daily mean windspeed, w kn, and the daily mean temperature, t C. A random sample of 9 consecutive days is taken from past records...
-
Suppose k(x) = f(g(h(x))). Given the table of values below, determine k' (1). g(x) h(x) f'(x) g'(x) h'(x) x f(x) 1 -6 -3 3 6 -6 -6 3 -3 4 1 -7 -2 5 4 -2 7 3 1 -7 -8
-
In a research study women with metastatic stomach cancer responded to the Symptom Distress Scale and the Profile of Mood States. A correlation coefficient was reported: r = 0.5, p = 0.03. How would...
-
Show that it is possible for (an and (bn both to diverge and yet for ((an + bn) to converge?
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
Compound A (C9H12) absorbed 3 equivalents of H2 on catalytic reduction over a palladium catalyst to give L (C9H18). On ozonolysis, compound A gave, among other things, a ketone that was identified as...
-
Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of H2 on catalytic reduction over a palladium catalyst. On ozonolysis, only two products are formed: oxalic acid (HO2CCO2H) and succinic...
-
Identify the reagents a?c in the following scheme: H.
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


