The trichloromethyl anion, :CCl 3 , which is the conjugatebase anion of chloroform (HCCl 3 ),
Question:
The trichloromethyl anion, –:CCl3, which is the conjugatebase anion of chloroform (HCCl3), is stabilized not only by the polar effect of the chlorines but also by resonance: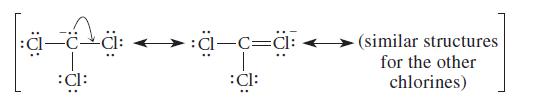
Show the orbital overlap between carbon and chlorine that is implied by the resonance structures.
Transcribed Image Text:
apa aca :cl: :CI: (similar structures for the other chlorines)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The chlorines provide empty 3dorbitals to overlap with the 2porb...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The pentadienyl radical, H2C == CH -- CH == CH -- CH2, has its unpaired electron delocalized over three carbon atoms. (a) Use resonance forms to show which three carbon atoms bear the unpaired...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Dudley B. Durham, Jr. and Barbara L. Durham, husband and wife, and their farming and trucking corporations, Double D Farms, Inc. and L.B. Trucking, Inc petitions on December 20, 1983 * * *. On March...
-
Question 5 [ 4 points ] In the table shown below is accounting equation information as it applies to Second Time Around Clothing. Calculate the missing amounts assuming that a . Assets decreased by $...
-
Brad Company sells ships. Each ship sells for over $25 million. Brad never starts building a ship until it receives a specific order from a customer. Brad usually takes about four years to build a...
-
A mountain-climbing expedition establishes two intermediate camps, labeled A and B in the drawing, above the base camp. What is the magnitude (r of the displacement between camp A and camp B? 4900 m...
-
1 Read Appendix A, Building an Effective Marketing Plan. Then write a 600-word executive summary for the Paradise Kitchens marketing plan using the numbered headings shown in the plan. When you have...
-
Mabel and Alan, who are in the 35% tax bracket, recently acquired a fast-food franchise. Both of them will work in the business and receive a salary of $175,000. They anticipate that the annual...
-
question: 5 year lease, first payment is $10,000 at end of the first period, payment increases by 5% each time and interest rate is 7%. Determine present value of lease and write out the amortization...
-
(a) Describe the bond for the double bond in Ph 3 P=CH 2 ; that is, what orbitals are involved on carbon and phosphorus? (b) Draw a resonance structure for the compound in part (a) that maintains...
-
Buster Bluelip, a student repeating organic chemistry for the fifth time, has observed that alcohols can be converted into alkyl bromides by treatment with concentrated HBr. He has proposed that, by...
-
The following three Lewis structures can be drawn for N2O: (a) Using formal charges, which of these three resonance forms is likely to be the most important? (b) The N-N bond length in N2O is 1.12...
-
Can anyone explain me how to calculate the ROI using the HISTORICAL COST NBV, the formula my instructor wants me to use is ADJ CF - HIST DEP /ASSETTOTAL - ACC DEP. And for the ROI of CURRENT COST NBV...
-
Consider the circuit to the right 3. If the total voltage supply in the circuit is 120V, and each resistor has a resistance of 400, what will the current read on each ammeter? |1= 12= 3 = 4. What...
-
1. The theory predicts the proportion of beans, in the four groups A, B, C and D should be 9:3:3:1. In an experiment among 1600 beans, the numbers in the four groups were 882, 313, 287 and 118. Does...
-
Would you recommend criminal charges in this case ( the screenshots below) and, if so, exactly which statutes against which person? Explain your reasoning (how the elements of the crime are met or...
-
check if each transaction is placed in the right place in each of the reports below and if there are any other mistakes in the different accounts after the first image which is a description of the...
-
Convert the following degree measures to radians (leave in your answer). (a) 30 (b) 45 (c) - 60 (d) 240 (e) -370 (f) 10
-
On October 1, 2014, the Dow Jones Industrial Average (DJIA) opened at 17,042 points. During that day it lost 237 points. On October 2 it lost 4 points. On October 3 it gained 209 points. Deter-mine...
-
One of the steps in the biosynthesis of uridine mono phosphate is the reaction of aspartate with carbamoyl phosphate to give carbamoyl aspartate followed by Cyclization to form dihydroorotate....
-
Order the following monomers with respect to (heir expected reactivity toward cationic polymerization, and explain your answer: H2C = CHCH3, H2C = CHCl, H2C = CHC6H5, H2C = CHCO2CH3
-
Order the following monomers with respect to their expected reactivity toward anionic polymerization, and explain your answer: H2C = CHCH3, H2C = CHC N, H2C = CHC6H5
-
Maddox Resources has credit sales of $ 1 8 0 , 0 0 0 yearly with credit terms of net 3 0 days, which is also the average collection period. Maddox does not offer a discount for early payment, so its...
-
Selk Steel Co., which began operations on January 4, 2017, had the following subsequent transactions and events in its long-term investments. 2017 Jan. 5 Selk purchased 50,000 shares (25% of total)...
-
Equipment with a book value of $84,000 and an original cost of $166,000 was sold at a loss of $36,000. Paid $100,000 cash for a new truck. Sold land costing $330,000 for $415,000 cash, yielding a...

Study smarter with the SolutionInn App


