The UV spectrum of p-nitrophenol in aqueous solution is shown in Fig. P18.59 (spectrum A). When a
Question:
The UV spectrum of p-nitrophenol in aqueous solution is shown in Fig. P18.59 (spectrum A). When a few drops of concentrated NaOH are added, the solution turns yellow and the spectrum changes (spectrum B). On addition of a few drops of concentrated acid, the color disappears and spectrum A is restored. Explain these observations.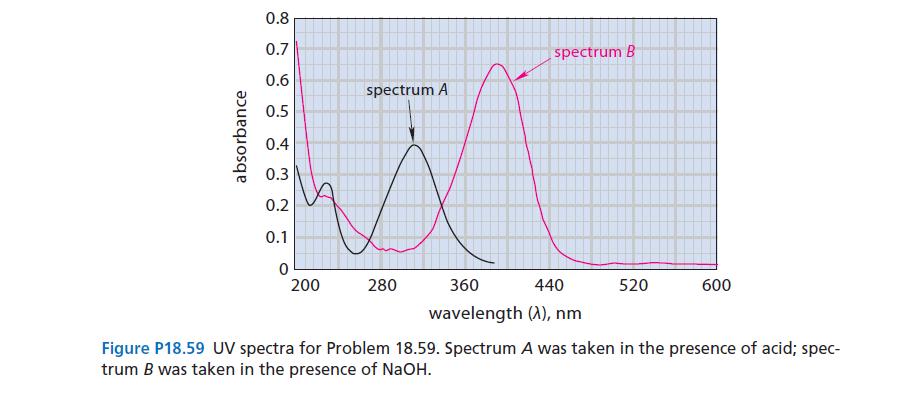
Transcribed Image Text:
absorbance 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 200 spectrum A 280 spectrum B 360 440 wavelength (A), nm 520 600 Figure P18.59 UV spectra for Problem 18.59. Spectrum A was taken in the presence of acid; spec- trum B was taken in the presence of NaOH.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Because NaOH can ionize a phenol particularly a relatively aci...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A student was studying terpene synthesis, and she wanted to make the compound shown here. First she converted 3-bromo-6-methylcyclohexene to alcohol A. She heated alcohol A with sulfuric acid and...
-
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer...
-
The UV spectrum of an unknown compound shows values of λmax at 225 nm (ε = 10,000) and at 318 nm 1e = 402. The mass spectrum shows a molecular ion at m/z 96 and a prominent...
-
1. Enis falsely accuses Monalisa of stealing from Island Tours, Inc., their employer. Enis's statement is defamatory only if a. a third party hears it. b. Monalisa has not been caught. c. the...
-
Roy Akins was the accounting manager at Zelco, Inc., a tire manufacturer, and he played golf with Hugh Stallings, the CEO, who was something of a celebrity in the community. The CEO stood to earn a...
-
The income statement of Amelia Enterprises Inc. for the year ended December 31, 2015, is as follows: Instructions Using the information from Problem 16-2A and the additional information provided,...
-
What is the role of Government Business linkages in creating emerging market challengers? Do you think that emerging and transition economies can move to a more hands-off approach with time? LO.1
-
The Dynaco Manufacturing Company produces a product in a process consisting of operations of five machines. The probability distribution of the number of machines that will break down in a week...
-
En un sistema de costeo por rdenes de trabajo, el uso de materiales directos que se compraron previamente se registra como dbito a: un Inventario de Materias Primas. b Inventario de productos en...
-
Phenols, like alcohols, are Brnsted bases. (a) Write the reaction in which the oxygen of phenol reacts as a base with the acid H 2 SO 4 . (b) On the basis of resonance and polar effects, decide...
-
Contrast the reactivities of cyclohexanol and phenol with each of the following reagents, and explain. (a) Aqueous NaOH solution (b) NaH in THF (c) Triflic anhydride in pyridine, 0 8C (d)...
-
How do borrowers and lenders differ in their requirements? Can banks reconcile these differences?
-
How do transnational organizations and agreements influence national sovereignty and political autonomy ?
-
How do individuals reconcile the tension between rational deliberation and emotional impulses when making consequential decisions amidst volatile environments, and to what extent does the phenomenon...
-
Watch the video clip below; https://www.youtube.com/watch?v=sE6Ox3ikCMU 1. Do you think that 'Rick and Morty' was a good choice? Justify your answer. 2. Do you think that this campaign will work for...
-
by the hypothesis that we want to do descriptive method, and quantative research in Tim hortons company, the question is A convincing closing statement, including that you'll develop your research...
-
Bottom of Form Why do you think ethics is important in healthcare management? What do you see as the biggest risks and temptations? How is your INTEGRITY a core principle in your professional ethical...
-
(a) Prove that every 3 3 proper orthogonal matrix has +1 as an eigenvalue. (b) True or false: An improper 3 3 orthogonal matrix has -1 as an eigenvalue.
-
Q1) What is the a3 Value Q2) What is the a7 Value Q3) What is the a4 Value Q4) What is the b3 Value Q5) What is the b2 Value Q6) What is the sign of 2nd constraint? A pastry chef at a bakery wants to...
-
A commercial synthesis of folic acid consists of heating the following three compounds with aqueous sodium bicarbonate. Propose reasonable mechanisms for the reactions that lead to folic acid. Hint:...
-
Outline a preparation of benzylamine using the Gabriel synthesis.
-
Give structures for compounds R-W: CHal H.O) Ag20 H20 N-Methylpiperidine- R (CH16NI) 0-5 (C,HyNO) at CHal H2O
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


