What product(s) are formed when a Grignard reagent prepared from each of the following alkyl halides is
Question:
What product(s) are formed when a Grignard reagent prepared from each of the following alkyl halides is treated with D2O?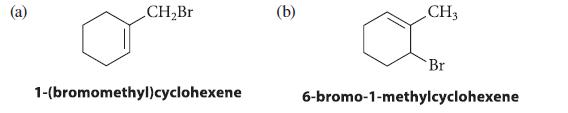
Transcribed Image Text:
CH₂Br 1-(bromomethyl)cyclohexene (b) CH3 Br 6-bromo-1-methylcyclohexene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
b The initially formed Grignard reagent undergoes a rapid al...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What product(s) are formed when a Grignard reagent prepared from the following alkyl halides is treated with D20? CH3 6-bromo-1-methylcyclohexene
-
Predict the major product that is obtained when each of the following alkyl halides is treated with potassium tert- butoxide. Explain your reasoning. (a) -Br (b) OCH3 T CH-CHI
-
What substitution and elimination products (if any)might be obtained when each of the following alkyl halides is treated with sodium methoxide in methanol? (a) methyl iodide (b)...
-
What favors the formation of continuous (dense) cleavage?
-
Working backward from changes in the Buildings and Equipment account The comparative balance sheets of American Airlines show a balance in the Buildings and Equipment account at cost year-end of...
-
Atoms can be cooled to incredibly low temperatures by letting them interact with a laser beam. Various novel quantum phenomena appear at these temperatures. What is the rms speed of cesium atoms that...
-
Explain why the following statement is incorrect: The probability of rain tomorrow is 150%.
-
Le Monde Company is a manufacturer of chemicals for various purposes. One of the processes used by Le Monde produces HTP3, a chemical used in hot tubs and swimming pools; PST4, a chemical used in...
-
Kenny wants to save $50,000 for an initial investment toward the purchase of a home in 5 years.What must he save at the beginning of each quarter if he can earn 9% compounded quarterly?
-
In each case, give the structure of a starting material that would give the product shown by MnO 2 oxidation @ CH=O (b) O (c) HOCH-
-
What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CCl 4 in the presence of light and peroxides? Explain your answers. (a) cyclohexene (b)...
-
EnCal is a small, California-based power company specializing in power generation methods that use clean-burning fuels and renewable natural resources. However, due to Californias complex and...
-
Brian is considering increasing the length of the cryptographic keys used by his organization. If he adds 8 bits to the encryption key, how many more possible keys will be added to the key space for...
-
Business law SECHON A [100 Marks] Read the scenario below then answer the questions that follow. Contracts are of critical importance especially in daily commercial and business transactions....
-
You may assume that the production costs to the winery are the same for each of the possible wines, despite the differences in volumes with some of the possible wines. Thus maximizing revenue will be...
-
You encounter a split system that uses R-22 refrigerant and observe the following refrigeration parameters from the unit's control display. The unit is operating in cooling mode. Suction pressure:...
-
A refrigerant at -20C is flowing through a 4" schedule 40 carbon steel pipe (inner diameter 102 mm, outer diameter 114 mm); the heat transfer coefficient for the refrigerant is 2500 W/m/K. It is...
-
The Securities and Exchange Commission (SEC) is an important governmental organization that exists primarily for the protection of the interests of investors in the U.S. securities markets. The SEC...
-
The comparative statements of financial position of Menachem NV at the beginning and end of the year 2019 appear below. Net income of ¬34,000 was reported, and dividends of ¬23,000 were paid...
-
How would the NMR spectrum of ethyl fluoride differ from that of ethyl chloride?
-
Describe in detail what changes you would expect to see in the NMR resonance of the methyl group as 1-chloro-l-methylcyclohexane is cooled from room temperature to very low temperature.
-
The proton-decoupled 13C NMR spectra of 3-heptanol (A) and 4-heptanol (B) are given in Fig. 13.22 on page 626. Indicate which compound goes with each spectrum, and explain your reasoning. Fig. 13.22...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...

Study smarter with the SolutionInn App


