When each of the compounds shown in Fig. P28.43 is heated in the presence of maleic anhydride,
Question:
When each of the compounds shown in Fig. P28.43 is heated in the presence of maleic anhydride, an intermediate is trapped as a Diels–Alder adduct. What is the intermediate formed in each reaction, and how is it formed from the starting material?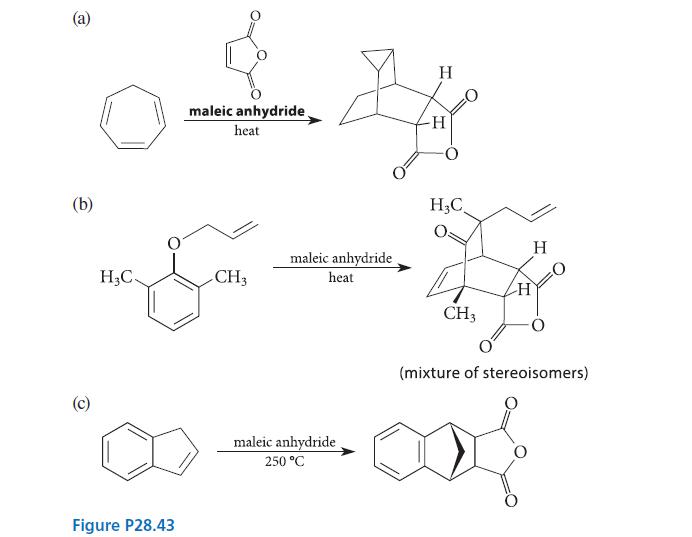
Transcribed Image Text:
(a) (b) (c) H₂C. maleic anhydride heat Son CH3 Figure P28.43 maleic anhydride heat maleic anhydride. 250 °C H H H₂C CH3 H H (mixture of stereoisomers)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a The approach to solving this type of problem is described in the solution to Pro...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many 13C NMR signals would you predict for each of the compounds shown in Problem 9.22?
-
What compounds would you expect to be formed when each of the following ethers is refluxed with excess concentrated hydrobromic acid? (a) (b) (c) (d) (THF) (1,4-dioxane)
-
What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CC14 in the presence of light and peroxides? Explain your answers. (a) cyclohexene (b)...
-
Nanette works for Piroz and is paid a basic wage of $1,000 a week. Piroz operates the following bonus scheme: (1) Each employee gets a bonus of $4 for every unit they produce in excess of 2,000 units...
-
You are considering investing the cash gifts you received for graduation in various stocks. You have received several annual reports of major companies. Required: For each of the following, indicate...
-
Conformity and Obedience Social Psychologists make a distinction between two types of social influence: Informational and Normative. Consider the role of both Informational and Normative influence in...
-
Following data are available from a record of a factory: Standard labour rate Rs 2 per hour Standard hours 2 per unit Actual labour rate Rs 2.25 per hour Actual units produced 1,000 units Actual...
-
Tel-Com Company, a telephone service and supply company, has just completed its fourth year of operations. The direct write-off method of recording bad debt expense has been used during the entire...
-
On January 2, 2017, Family Clothing Consignments purchased showroom fixtures for $16,000 cash, expecting the fixtures to remain in service for five years. Family has depreciated the foctures on a...
-
A compound A (C 11 H 14 O 3 ) is insoluble in base and gives an isomeric compound B when heated strongly. Compound B gives a sodium salt when treated with NaOH. Treatment of the sodium salt of B with...
-
Certain black bugs of the order Hemiptera, generally observed in the tropical regions of India immediately after the rainy season, give off a characteristic nauseating smell whenever they are...
-
a. Can the delta of a call option be greater than 1.0? Explain. b. Can it be less than zero? c. How does the delta of a call change if the stock price rises? d. How does it change if the risk of the...
-
Dr. Powers operates a single-provider family medical practice. One medical assistant handles appointments, basic bookkeeping functions, and assists with medical records. Two additional medical...
-
Quiz 6 Fall 2019 - MGCR-211-001/002/003 edugen.wileyplus.com WileyPLUS Financial Accounting, Seventh Canadian Edition by Kimmel, Weygandt, Kieso, Trenholm, Irvine, and Burnley Help | System...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
4 Listen Using the DCF approach yields the value of the company as a whole. How would one refine this to determine the value of a share of stock? 1) Divide the company value by total assets. 2)...
-
The "is" or "is not" test established in McPhail v. Doulton (1971) for discretionary trusts creates more problems than it resolves.' Critically evaluate this statement. requirement Table of content...
-
Genie in a Bottle Company (GBC) manufactures plastic two-liter bottles for the beverage industry. The cost standards per 100 two-liter bottles are as follows: Standard Cost per 100 Two-Liter Cost...
-
The power company must generate 100 kW in order to supply an industrial load with 94 kW through a transmission line with 0.09 resistance. If the load power factor is 0.83 lagging, find the...
-
The nucleosides shown in Figs. 25.4 and 25.5 are stable in dilute base. In dilute acid, however, they undergo rapid hydrolysis yielding a sugar (deoxyribose or ribose) and a heterocyclic base. (a)...
-
Basing your answer on reactions that you have seen before, propose a likely mechanism for the condensation reaction in the first step of the preceding uridine synthesis.
-
(a) What kind of linkage is involved in the acetonide group of the protected nucleoside, and why is it susceptible to mild acid-catalyzed hydrolysis? (b) How might such a protecting group be...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


