When the diethyl ester of a substituted malonic acid is treated with sodium ethoxide and urea, Veronal,
Question:
When the diethyl ester of a substituted malonic acid is treated with sodium ethoxide and urea, Veronal, a barbiturate, is formed (see Fig. P22.84). (Barbiturates are hypnotic drugs; some are actively used in modern anes-thesia.) Using the curved-arrow notation, give a mechanism for the Veronal synthesis.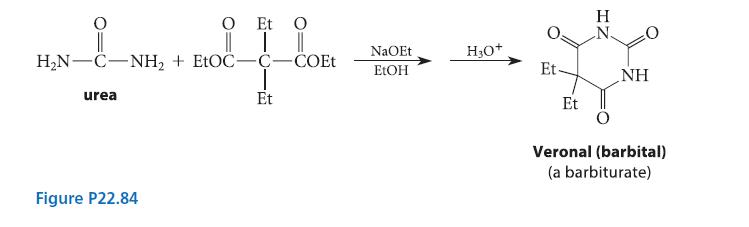
Transcribed Image Text:
O Et O _i_ || HN-C−NH, + EtOC—C−COEt Hi urea Et Figure P22.84 NaOEt EtOH H₂O+ Et Et IZ H NH Veronal (barbital) (a barbiturate)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
This reaction is essentially a type of crossedClaisen conden...View the full answer

Answered By

Shikha SharmaThakur
My teaching method is primarily focused on explaining practical scenarios which helps in quick and effective understanding.
I begin with basic concepts, followed by intensive practice in tutorials and examples.
I usually prefer quick tests to know students understanding of the subject.
I design my own examples in various stages of difficulty levels to help in students understanding in addition to the text book questions
Mathematics is to solve a task with a set of rules. I provide quick notes and rules to be refereed that can be helpful to solve questions of any difficulty level
I believe learning is better if you can visualize it, and hence in addition to providing real world examples, I prefer teaching using graphs and plots for enhanced understanding of the subject
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the mechanistic symbols (SN1, SN2, E1, E2) that are most consistent with each of the following statements: (a) Methyl halides react with sodium ethoxide in ethanol only by this mechanism. (b)...
-
Give the mechanistic symbols (SN1, SN2, E1, E2) that are most consistent with each of the following statements: (a) Methyl halides react with sodium ethoxide in ethanol only by this mechanism. (b)...
-
When cis-1-bromo-4-tert-butylcyclohexane is treated with sodium ethoxide in ethanol, it reacts rapidly; the product is 4-tert-butylcyclohexene. Under the same conditions, trans-...
-
A newly issued 20-year maturity, zero-coupon bond is issued with a yield to maturity of 8% and face value $1,000. Find the imputed interest income in the first, second, and last year of the bonds...
-
Explain why the Merchandise Inventory account will usually require adjustment at year-end.
-
Write an article on "Buddhism religion".
-
Why do foreign businesses hesitate to invest in Russia? LO.1
-
Bruno Industries expects credit sales for January, February, and March to be $200,000, $260,000, and $310,000, respectively. It is expected that 70% of the sales will be collected in the month of...
-
Sam Nash is the head of new product development of Forever Young (FY). Nash is currently considering Enhance, which would be FY's next major product in its beauty/cosmetics line, and its estimated...
-
Using the curved-arrow notation, provide mechanisms for each of the reactions given in Fig. P22.87. H3C C-CH3 (c) (b) BrCHCO,CH3 I (CH)3 T BrCHCO,CH3 2 COEt KCO3 COEt NaOEt + 2 NaH HO CH3 DMF HO,...
-
A useful diketone, dimedone, can be prepared in high yield by the synthesis shown in Fig. P22.83. Provide structures for both the intermediate A (a Michael-addition product) and dimedone, and give a...
-
Derive the equation of motion and natural frequency for the disk shown in Figure 2.55. Assume the disk is rolling without slip. y k eelle 16 G k eelle Xo Figure 2.55: Disk on an inclined plane...
-
As a new principal, I assigned a teacher to a different grade for the coming year. I did not expect to cause the anxiety it did. The teacher first came to me in tears and begged for her assignment to...
-
Peruse the following websites to learn about the different ways of categorizing leadership. 1. https://www.businessnewsdaily.com/9789-leadership-types.html 2....
-
Making Consumer Choices The Espresso Machine (25 points) In real life, you must often make choices about whether to buy something pre-made or make it yourself. There are many things to consider:...
-
1) Read over the article/case and summarize what it is referring to in your own words. 2) What type of leadership traits can you describe in the case study? Use materials both from the handout and...
-
After reading or watching, https://smallbusiness.chron.com/internal-analysis-important-80513.html https://www.indeed.com/career-advice/career-development/internal-analysis...
-
The matrix A = arises in the finite difference (and finite element) discretization of the Poisson equation on a nine point square grid. Solve the linear system A u = e5 using (a) Gaussian Elimination...
-
A line l passes through the points with coordinates (0, 5) and (6, 7). a. Find the gradient of the line. b. Find an equation of the line in the form ax + by + c = 0.
-
Write structures for the major organic products from the following reactions. Show stereoisomers where applicable. (a) (b) (c) (d) C2 (1 equiv.) HBr (excess) (1) NaNH2 (2) PhCH2Br (1) 03 (2) HOAc
-
Show how 1-butyne could be synthesized from each of the following: (a) 1-Butene (b) 1-Chlorobutane (c) 1-Chloro-1-butene (d) 1, 1-Dichlorobutane (e) Ethyne and ethyl bromide
-
Starting with 2-methylpropene (isobutylene) and using any other needed reagents, outline a synthesis of each of the following: (a) (b) (c) (d) OR Br CI HO
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


