Write a curved-arrow mechanism for the reaction in Eq. 19.62. (This is a base-catalyzed process; the base
Question:
Write a curved-arrow mechanism for the reaction in Eq. 19.62. (This is a base-catalyzed process; the base is sodium acetate, Na+ AcO–.)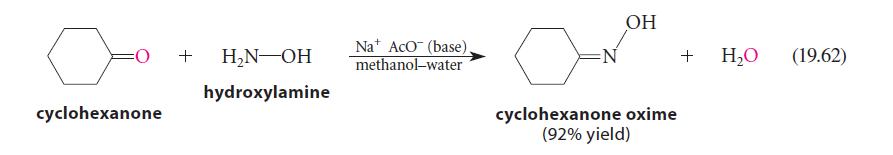
Transcribed Image Text:
cyclohexanone + H₂N-OH hydroxylamine Na AcO (base). methanol-water N OH cyclohexanone oxime (92% yield) + H₂O (19.62)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
First the carbinolamine intermediate is formed This ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
2. Draw the curved arrow mechanism for the reaction between 2-methoxyphenoxide and 3-chloro-1,2-propanediol, in the guaifenesin synthesis. Be sure to clearly show the bimolecular transition state.
-
The total amount paid in by__________for the shares they purchase is described as common stock. Select answer from the options below creditors bondholders employees stockholders
-
Write equations describing the addition-elimination mechanism for the reaction of hexafluorobenzene with sodium methoxide, clearly showing the structure of the rate-determining intermediate.
-
QI: Obtain the closed loop transfer function for the system whose block diagram is shown in fig.(1) 3 Y(s) 2 +. X(s) s+2 s+2 s+2 Fig. (1)
-
This exercise continues the Lawlor Lawn Service, Inc., situation from Exercise 2-61 of Chapter 2. Start from the trial balance and the posted T-accounts that Lawlor Lawn Service prepared at May 31,...
-
Tyndal Company had the following items that required adjustment at December 31, 2019. a. Purchased equipment for $40,000 on January 1, 2019. Tyndal estimates annual depreciation expense to be $3,100....
-
What are the various types of perceived risk?
-
The spreadsheet file "Chapter 5 Problem 3.xlsx" (to find the student spreadsheets for Financial Analysis with Microsoft Excel, seventh edition, go to www.cengagebrain.com) contains monthly total...
-
Please help Required Information [The following information applies to the questions displayed below.] Henna Company produces and sells two products, Carvings and Mementos. It manufactures these...
-
Draw the structure of (a) The oxime of acetone; (b) The imine formed in the reaction between 2-methylhexanal and ethylamine (EtNH 2 ).
-
Outline a synthesis of the following compound from p-bromoacetophenone and any other reagents. HC-C- -C-CH3
-
How can sentiment analysis be used for brand management?
-
How do you manage global and international teams? What would you do different?
-
Your friend is super excited about the results of their study! They examined whether different parenting styles [A] resulted in differences in anxiety levels among children. The different levels (a)...
-
Test the series for convergence or divergence. n=1 e1/n 78 O convergent O divergent
-
How do employees perceive the organization's vision and mission, and to what extent do these perceptions influence their commitment to the organization ?
-
In the table below which shows class taken and grade achieved, find the probability that a student selected takes Stat or receives a B grade. Round your answer to three decimal places 40 70 70 50 40...
-
Determine whether the following symmetric matrices are positive definite by computing their eigenvalues. Validate your conclusions by using the methods from Chapter 4. (a) (b) (c) (d) 3 2 011 121 110...
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
Show how pentanoic acid can be prepared from each of the following: (a) 1-Pentanol (b) 1-Bromobutane (two ways) (c) 5-Decene (d) Pentanal
-
The active ingredient of the insect repellent Off is N, N-diethyl-m-toluamide, m-CH3C6H4CON (CH2CH3)2. Outline a synthesis of this compound starting with 3-methylbenzoic acid (m-toluic acid).
-
Starting with benzene and succinic anhydride and using any other needed reagents, outline a synthesis of 1-phenylnaphthalene.
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


