A compound with molecular formula C 9 H 10 O exhibits the following spectra ( 1 H
Question:
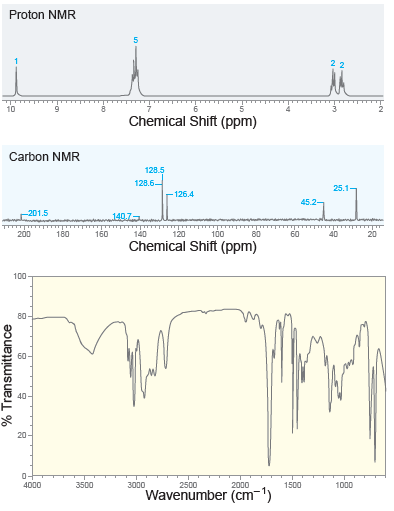
Transcribed Image Text:
Proton NMR 10 Chemical Shift (ppm) Carbon NMR 128.5 128.6- 25.1- 126.4 201.5 140.7 160 140 120 100 200 180 80 60 40 20 Chemical Shift (ppm) 100 80 - 60- 40- 20- 0- 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-') % Transmittance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A compound with molecular formula C 9 H 10 O exhibits a strong signal at 1687 cm 1 in its IR spectrum. The 1 H and 13 C NMR spectra for this compound are shown below. Identify the structure of this...
-
Identify the compound with molecular formula C8H10O that gives the IR and 1H NMR spectra shown in Figure 14.23. 23 16 27 2 29 35 13 14 15 16 800 200 2400 200 0 6 (ppm) frequency
-
Propose a structure for a compound with molecular formula C 3 H 8 O that exhibits the following 1 H NMR and 13C NMR spectra: Proton NMR 0.5 5.0 4.5 4.0 3.5 3.0 2.0 1.5 1.0 25 Chemical shift (ppm)...
-
Every real number is either a/an number or a/an_______ number.
-
Explain the differences among the three major forms of bankruptcy: Chapter 7, Chapter 11, and Chapter 13.
-
Use the Divergence Theorem to evaluate and find the outward flux of F through the surface of the solid bounded by the graphs of the equations. Use a computer algebra system to verify your results....
-
Explain the advantages and disadvantages of alternative advertising media.
-
Notes Payable On December 1, 2007 Insto Photo Company purchased merchandise, invoice price $25,000, and issued a 12%, 120-day note to Ringo Chemicals Company. Insto uses the calendar year as its...
-
Score: orTp S9-9 (similar to) Question Help * ABC Cabering Service purchased equipment on January 1, 2018, for $41,852 Suppose ABC Catering Service sold the equipment for $30,000 on December 31,...
-
Suppose that a students course marks for quiz 1, quiz 2, test 1, test 2, and the final exam are 70, 85, 80, 75, and 90, respectively. Write his marks as a column vector.
-
Solid methanol in thermal contact with the surroundings is reversibly melted at the normal melting point at a pressure of 1 atm. Are S, S surroundings , and S total positive, negative, or zero?...
-
Can incandescent lighting be regarded as an example of cogeneration during the heating season? In a season where air conditioning is required?
-
The average price of a movie ticket in 2004 was $6.21. In 2016, the average price was $8.65. Find and interpret the average rate of change in the price of a movie ticket per year to the nearest cent.
-
PART 1: DIGITAL TECHNOLOGY: Describe the key digital technology groups studied in this course and include a discussion of two examples for each group. PART 2: SOCIAL MEDIA: As studied in this course,...
-
Doing a strategic analysis of GraceKennedy Limited, What is the current level of its economic performance, an indication of the factors responsible for the current performance and recommendations for...
-
Dynamic capability is the ability for change and manage corporate learning. It allows an enterprise to adapt, develop and respond to future opportunities and discontinuous technologies. Innovation...
-
What potential solutions can organizations try to help support the adoption of a CDSS? In other words, what are some ways an organization can address the factors of implementation obstruction that...
-
Identify and briefly describe and discuss the three most important factors in building and maintaining trust among virtual global team members. Include in your discussion how you can leverage these...
-
Use synthetic division to divide f(x) = x 3 - 4x 2 + x + 6 by x + 1. Use the result to find all zeros of f.
-
Stephen Schor, an accountant in New York City, advised his client, Andre Romanelli, Inc., to open an account at J. P. Morgan Chase Bank, N.A., to obtain a favorable interest rate on a line of credit....
-
(a) Give the structure of the S*2 reaction product between ethyl iodide and potassium acetate. H,C-C potassium acetate
-
We can conceive of a stepwise version of the SN2 reaction consisting of a Lawis acid-base dissociation followed by a Lewis acid-base association. (Nuc:- = a nucleophile.) (a) Why should the stepwise...
-
We can conceive of a stepwise version of the SN2 reaction consisting of a Lawis acid-base dissociation followed by a Lewis acid-base association. (Nuc:- = a nucleophile.) (a) Why should the stepwise...
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


