AIBN is an azo compound (a compound with a N=N double bond) that is often used as
Question:
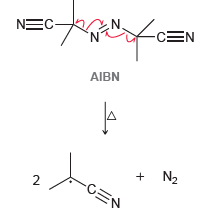
(a) Give two reasons why these radicals are so stable.
(b) Explain why the following azo compound is not useful as a radical initiator:
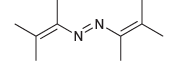
Transcribed Image Text:
-CEN NEC- AIBN + N2 -CEN 2. .N. N=N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
a These radicals are tertiary and they ...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give an example of each of the following: a. A primary amine b. A cyclic tertiary amine c. A secondary aromatic amine d. A quaternary ammonium salt e. An aryldiazonium salt f. An azo compound g. A...
-
An organic compound with the molecular formula C4H10O3 shows properties of both an alcohol and an ether. When treated with an excess of hydrogen bromide, it yields only one organic compound, 1,...
-
An optically active monoterpene (compound A) with molecular formula undergoes catalytic hydrogenation to form an optically inactive compound with molecular formula C10H20O (compound B). When compound...
-
The Jasper SkyTram transports passengers to the top of Whistler Mountain in the Canadian Rockies where there are spectacular views, boardwalks, and hiking trails. The SkyTram travel time (in minutes)...
-
What is the maximum rise in body temperature that analysts should allow?
-
You are waiting for a UPS package, and you want to know if it is in the mailroom yet. For each of the communication situations, select the most appropriate channel for transmitting the message. Oral...
-
What are the four assumptions made about the probability distribution of e in regression? Illustrate the assumptions with a graph. LO9
-
Three different plans for financing a $10,000,000 corporation are under consideration by its organizers. Under each of the following plans, the securities will be issued at their par or face amount,...
-
3. On January 2, 2019, Worth Co. issued at par $1,000,000 of 7% convertible bonds. Each $1,000 bond is convertible into 20 shares of common stock. No bonds were converted during 2019. Worth had...
-
Computer control of a robot to spray-paint an automobile is accomplished by the system shown in Figure DP5.8(a) [7]. We wish to investigate the system when K = 1, 10, and 20. The feedback control...
-
When ethylbenzene is treated with NBS and irradiated with UV light, two stereoisomeric compounds are obtained in equal amounts. Draw the products and explain why they are obtained in equal amounts....
-
Examine the following figure and fill in the information below. a. The grape sugar level starts at g and ends at g. b. The yeast population reaches its highest level of approximately on Day . c. The...
-
The accountant of a goldmining company in Western Australia has to make a decision about whether to record an accounting transaction or not. The goldmining company discovered an extremely rich seam...
-
Pomerantz in Chapter 8 discussed the expectation that the years leading up to 2030 Group of answer choices would be years of accelerating progress, a trend derailed by war, pandemic, economic...
-
A Mississippi chicken processing plant fired most of its remaining workers after nearly 100 accused of immigration violations were arrested last week, witnesses said, an indication that the crackdown...
-
You have added 15 songs to a Spotify playlist. You have 3 classic rock songs, 7 pop songs, and 5 country songs. You can play 4 songs on your walk to school. What is the probability that you hear 2...
-
a b Solve a) (725.25)10=(?)2=(?)16 b) (111100111110001)2= (?) 8 = (?) 16 Build the equation Y=AB+ CD + E to realize using a) NAND Gates b) NOR Gates Construct and describe Full Adder with neat logic...
-
With such a high base rate, you are confident about the chance of hiring and have posted the job ad based on a prior job analysis. Listed below are the final applicants and their profile of four key...
-
The following exercises are of mixed variety. Factor each polynomial. 1 - x 16
-
In Problem 8.43, determine the smallest value of for which the rod will not fall out of the pipe. IA -3 in.-
-
Calculate the formal charges on all of the atoms except hydrogen's, in these compounds: a) H-N-N=N: c) H-C-N=N: H e) H H-=C-H b) H-N-N-N: HO: H-C-C-C 1 H d) f) H H-B-H T H
-
Explain which of the two following structures would be more stable. Explain whether they represent isomers or are resonance structures. HIN: H N-H H :0: N-H HIN
-
Draw a Lewis structure for carbon monoxide (CO). Calculate the formal charges on the atoms and comment on the stability of this compound.
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


