At high temperatures, alkanes can undergo dehydrogenation to produce alkenes. For example: This reaction is used industrially
Question:
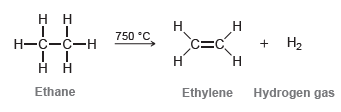
This reaction is used industrially to prepare ethylene while simultaneously serving as a source of hydrogen gas. Explain why dehydrogenation only works at high temperatures.
Transcribed Image Text:
Н. нн 750 °C. Н Н—с—с—н c=C + H2 Н Ethane Ethylene Hydrogen gas
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
A reaction is only favorable if G is negative Recall that G has two components H and TS ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At high temperatures, a dynamic equilibrium exists between carbon monoxide, carbon dioxide, and solid carbon. At 850oC, Kc is 0.153. a. What is the value of Kp? b. If the original reaction system...
-
At high temperatures, a dynamic equilibrium exists between carbon monoxide, carbon dioxide, and solid carbon. At 900oC, Kc is 0.238. a. What is the value of Kp? b. Some carbon dioxide is added to the...
-
Reverse Diels-Alder reactions can occur at high temperatures. Why are high temperatures required?
-
Tonight at 7pm a professional basketball game featuring the Golden State Warriors and the Washington Wizards will be played at the Chase Center, an indoor arena in the Mission Bay neighborhood of San...
-
Create a fictional company and present a marketing campaign to brand that company. Make sure that you give me some background as to what the company sells and who you are trying to reach; your...
-
A 2-lb block rests on the smooth semicylindrical surface k at A. An elastic cord having a stiffness of k = 2 lb/ft is attached to the block at B and to the base of the semicylinder at C. If the block...
-
The ascertainment of cost after it has been incurred is (a) Job costing (b) Batch costing (c) Absorption costing (d) Historical costing
-
Describe the even planning process and explain why it is helpful.
-
Selected current year - end financial statements of Cabot Corporation follow. ( All sales were on credit; Compute the following: ( 1 ) current ratio, ( 2 ) acid - test ratio, ( 3 ) days' sales...
-
Referring to Exercise 3.39 find (a) f(y\2) for all values of y; (b) P(Y = 0 | X = 2).
-
Identify the reagents you would use to convert 1-pentene into a geminal dibromide (geminal indicates that both bromine atoms are connected to the same carbon atom).
-
Identify the reagents you would use to convert methylcyclohexane into each of the following: (a) A 3 alkyl halide (b) A trisubstituted alkene (c) A 2 alcohol (d) 3-methylcyclohexene
-
Hurst Company has variable costs of \(\$ 26\) per unit and a contribution margin ratio of 35 percent. Compute the selling price per unit.
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{D}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
Draw a line graph for each data set in Problems 36-39. Data set A Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 25 16 25 25 14 18 1 2 2 2...
-
For each of the angles shown: (i) Estimate its size (ii) Measure it and check how good your estimate was. Aim for your estimate to be within 10 of the actual angle. a. b. c. d. e. f.
-
For the quasispin model of Problem 31.1 , find the eigenvalues of $s_{0}^{(m)}$ for the levels labeled by $m$. Show that the system has a total quasispin $S$ that is the vector sum of quasispins for...
-
A sole proprietorship was started on January 1, 2005, when it received \($60,000\) cash from Mark Pruitt, the owner. During 2005, the company earned \($40,000\) in cash revenues and paid \($19,300\)...
-
Solve each logarithmic equation in Exercises 4992. Be sure to reject any value of x that is not in the domain of the original logarithmic expressions. Give the exact answer. Then, where necessary,...
-
Which of the following raises the credibility of areport? Which of the following raises the credibility of a report? Multiple Choice avoiding predictions avoiding the use of cause-effect statements...
-
How much is the reaction rate for these reactions increased or decreased if the concentration of hydroxide ion is doubled? If the concentrations of the both the alkyl chloride and hydroxide ion...
-
Show how these compounds could be prepared from alkylhalides: SCH, OCCH3 b) OCH3 a) c) Two methods Two methods
-
Show how these products could be synthesized from the indicated starting material. More than one step may be necessary. Make sure that the product has the stereo chemistryshown. Starting material...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


