Show how these products could be synthesized from the indicated starting material. More than one step may
Question:
Show how these products could be synthesized from the indicated starting material. More than one step may be necessary. Make sure that the product has the stereo chemistryshown.
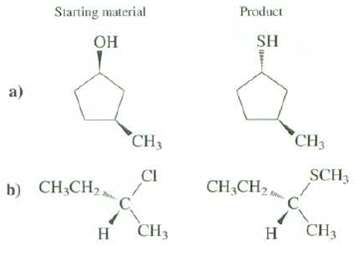
Transcribed Image Text:
Starting material Product SH Он a) CH3 CH3 CI SCH , CH,CH2 b) CH,CH, CH3 CH3 н н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (21 reviews)
a b OH TSCI pyridine CH3 CI CH...View the full answer

Answered By

Sumit kumar
I am an experienced online essay writer with a thorough understanding of any curriculum.and subject expert at Chegg for mathematics, CS subjects..
4.90+
5+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show how Tagamet could be synthesized from the indicated starting materials. CH2OH CH CH SCH2CH2NH NHCH N CH CHS SCH CH3 +CH:NH2
-
Show how each of the following products could be synthesized from butanal: (a) 2-Ethyl-3-hydroxyhexanal (b) 2-Ethylhex-2-en-1-ol (c) 2-Ethylhexan-1-ol (d) 2-Ethylhexane-1, 3-diol (the insect...
-
Show how ethyl phenyl ketone (C6H5COCH2CH3) could be synthesized from each of the following: (a) Benzene (b) Benzonitrile, C6H5CN (c) Benzaldehyde
-
Problem 1: The three velocities shown would indicate that the body is not rigid. Given that the body is known to be rigid, one of the three velocities is clearly incorrect. Draw all lines and take...
-
We don't need a product costing system. We are a small manufacturer with a just-in-time approach so that our inventories ore minimal. We have no influence over product price; whatever the big fellows...
-
The individual financial statements for Gibson Company and Keller Company for the year ending December 31, 2018, follow. Gibson acquired a 60 percent interest in Keller on January 1, 2017, in...
-
Explain why we might not want to remove a variable just because it is highly correlated with another variable. EXERCISES 53 HANDS-ON ANALYSIS Use the churn data set14 on the book series web site for...
-
Suppose that in 2023 one- and two-year interest rates are 5.2% in the United States and 1.0% in Japan. The spot exchange rate is 120.22/$. Suppose that one year later interest rates are 3% in both...
-
The projected benefit obligation was $120 million at the beginning of the year. Service cost for the year was $8 million. At the end of the year, pension benefits paid by the trustee were $4 million...
-
Five clowns each have a red wig and a blue wig, which they are all equally likely to wear at any particular time. Find the probability that, at any particular time: a. Exactly two clowns are wearing...
-
Show how these compounds could be prepared from alkylhalides: SCH, OCCH3 b) OCH3 a) c) Two methods Two methods
-
Cyanide anion has two potential nucleophilic sites: the carbon and the nitrogen. Explain which site is expected to be the strongernucleophile. :c=N:
-
The authors of the paper Illicit Use of Psychostimulants among College Students (Psychology, Health & Medicine [2002]: 283287) surveyed college students about their use of legal and illegal...
-
Sample grade point averages for ten male students and ten female students are listed. Males 2.4 3.7 3.8 3.9 Females 2.8 3.7 2.1 3.9 2.8 2.6 3.6 3.3 4.0 1.9 3.6 4.0 2.0 3.9 3.7 2.3
-
Fill in the columns in the following table. What quantity should a profit-maximizing firm produce? Verify your answer with marginal reasoning. 9 0 1 2 3 st 4 5 6 TFC $5 5 5 5 5 5 5 TVC $0 3 5 9 16 25...
-
Perform the experiments in Problems 48-51, tally your results, and calculate the probabilities (to the nearest hundredth). Flip three coins simultaneously 100 times, and note the results. The...
-
The following information is available for Spring Inc. and Winter Inc. at December 31, 2011: Required a. What is the accounts receivable turnover for each of the companies for 2011? b. What is the...
-
Margin of error = 0.5 g, standard deviation = 8.7 g
-
To discuss the practice of whistle-blowing and the conditions for its moral appropriateness
-
Reichenbach Co., organized in 2018, has set up a single account for all intangible assets. The following summary discloses the debit entries that have been recorded during 2018 and 2019. Instructions...
-
What is the probability of rolling a number less than 5 with 1 dice?
-
How could you prepare the following Cyclohexanone by combining a Stork enamine reaction with an intra molecular aldol condensation? (b) (c) (a) CH CH
-
The amino acid leucine is biosynthesized from a-ketoisovalerate by the following sequence of steps. Show the mechanism ofeach. Acetyl CoA COASH NAD+ NADH/H* CO2 "C 2-Isopropylmalate co2 ...
-
The Knoevenagel reaction is a carbonyl condensation reaction of an ester with an aldehyde or ketone to yield an ?, ??un-saturated product. Show the mechanism of the Knoevenagel reaction of diethyl...
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


