The Knoevenagel reaction is a carbonyl condensation reaction of an ester with an aldehyde or ketone to
Question:
The Knoevenagel reaction is a carbonyl condensation reaction of an ester with an aldehyde or ketone to yield an ?, ??un-saturated product. Show the mechanism of the Knoevenagel reaction of diethyl malonate with benzaldehyde.
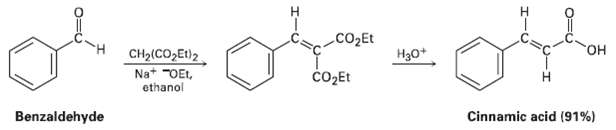
Transcribed Image Text:
cO2Et H30+ HO. CH2(CO,Et)2 čO2Et Na+ "OEt, ethanol Cinnamic acid (91%) Benzaldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Formation of the enolate of diethyl malonate is the ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Organic Chemistry questions
-
An ester is a compound formed by a condensation reaction between a carboxylic acid and an alcohol. Read the discussion of esters in Section 24.4 and then give an example of a reaction forming an...
-
The reaction of an aldehyde or ketone with a Grignard reagent is a nucleophilic addition to the carbon-oxygen double bond. (a) What is the nucleophile? (b) The magnesium portion of the Grignard...
-
Diethyl malonate is prepared commercially by hydrolysis and esterification of ethyl cyanoacetate. The preparation of ethyl cyanoacetate proceeds via ethyl chloroacetate and begins with acetic acid....
-
We look at the accumulated area beneath this curve, as in the definite integral as follows F(x) = f(t) dt -2 { F(x) = int_(-2)^x f(t) dt a) Use ordinary area formulas to compute each of the...
-
Give examples of actions that are substitutes in production. Give examples of actions that are compliments in production.
-
An object whose moment of inertia is 4.0 kg m 2 experiences the torque shown in Figure EX12.25. What is the objects angular velocity at t = 3.0 s? Assume it starts from rest. (Nm) 0+ -t (s) 3 FIGURE...
-
What your equity investors IRR would be based on the percentage of ownership you are planning to offer him, how much he is investing, and the cash flow you are projecting.
-
Thomas Consultants provided Bran Construction with assistance with implementing various cost-savings initiatives. Thomas' contract specifies that it will receive a flat rate of $50,000 and an...
-
Beginning work in process consists of 7,000 units, 55% complete. 50,000 units were started and ending work in process is 5,000 units, 25% complete. Normal spoilage is equal to 10% of the units...
-
For the figure shown, What is the maximum tensile stress in the beam? Hint: There will be two maximum moments (negative and positive moments), only considering the governing. 10KN/m 4m 1m E 8KN 125mm...
-
The amino acid leucine is biosynthesized from a-ketoisovalerate by the following sequence of steps. Show the mechanism ofeach. Acetyl CoA COASH NAD+ NADH/H* CO2 "C 2-Isopropylmalate co2 ...
-
The Darzens reaction involves a two-step, base-catalyzed condensation of ethyl chloroacetate with a ketone to yield an epoxy ester. The first step is a carbonyl condensation reaction, and the second...
-
If the initial tension in the belt is increased then the power transmitted by the belt (a) reduces (b) increases (c) remains same (d) depends on speed.
-
Consider the following C functions and assembly code: int fun4 (int *ap, int *bp) ( int a = *ap; int bbp; return a+b; }) pushl ebp movl esp, ebp int fun5 (int *ap, int *bp) { int bbp; *bp + *ap;...
-
The position of a particle moving along the x-axis is given by x(t) = = 4.2 2.5t m. (Assume t is in seconds.) (a) At what time (in s) does the particle cross the origin? 1.68 S (b) What is the...
-
2. Boxes A and B are being pulled to the right by a rope attached to box B. Box A sits on top of box B, and both boxes accelerate together to the right at a rate of 1.75 m/s. The masses and...
-
You bought a 15-kilogram sack of unshelled peanuts for your restaurant. You weigh the sack three times on a balance, with the following results: Trial Mass (kg) 1 15.02 2 15.49 3 15.91 The results...
-
Two hikers leave the same tent at a campground and go separate ways. One hiker walks 8 miles directly south to Ashville, and the other hiker walks 14 miles directly northwest (i.e., N45W) to...
-
Explain which leadership styleautocratic, bureaucratic, democratic, or laissez-fairewould be most effective when dealing with a hotel sales manager who you recently hired to boost corporate group...
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
Use the BornHaber cycle and data from Appendix IIB, Chapter 9, and this chapter to calculate the lattice energy of KCl. (H sub for potassium is 89.0 kJ/mol.) Substance Aluminum Al(s) Al(g) Al+(aq)...
-
Give the expected major product for each reaction, including stereochemistry where applicable. (a) but-1-ene + H2 / Pt (b) cis-but-2-ene + H2 / Ni (c) (d) + H/Pt + excess H,/Pt
-
One of the principal components of lemon grass oil is limoneneC10H16 When limonene is treated with excess hydrogen and a platinum catalyst, the product is an alkane of formula C10H20. What can you...
-
The chiral BINAP ligand shown in Figure 8-7 contains no asymmetric carbon atoms. Explain how this ligand is chiral.
-
The cost of partially completed goods at the end of the period would be Ending work in process inventory Cost of goods sold Beginning finished goods inventory Beginning work in process inventory
-
At a 3% (EAR) rate of interest, you will quadruple (increase four folds) your money in approximately ____ years.
-
Smile Company makes baked goods. The budgeted sales are $620,000, budgeted variable costs are $260,400, and budgeted fixed costs are $237,800. What is the budgeted operating income?

Study smarter with the SolutionInn App


