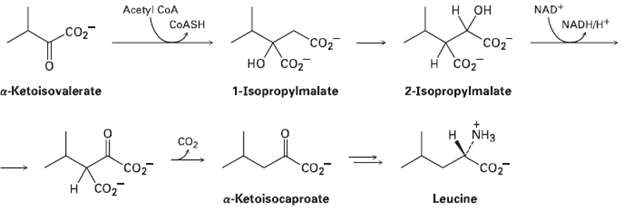
The amino acid leucine is biosynthesized from a-ketoisovalerate by the following sequence of steps. Show the mechanism
Question:
The amino acid leucine is biosynthesized from a-ketoisovalerate by the following sequence of steps. Show the mechanism ofeach.
Transcribed Image Text:
Acetyl CoA COASH NAD+ NADH/H* н он CO2 "Cот н со 2-Isopropylmalate co2 но со 1-Isopropylmalate a-Ketoisovalerate н. NHз co2 Co co2 co H CO2 a-Ketoisocaproate Leucine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
AHA 0 addition of acetyl COA CO2 CHCSCOA CO decarboxy...View the full answer

Answered By

Ashok Kumar Malhotra
Chartered Accountant - Accounting and Management Accounting for 15 years.
QuickBooks Online - Certified ProAdvisor (Advance - QuickBooks Online for 3 years.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The amino acid leucine is biosynthesized from ?-ketoisocaproate, which is itself prepared from a-ketoisovalerate by a multistep route that involves (1) reaction with acetyl CoA, (2) hydrolysis, (3)...
-
The amino acid cysteine, C3H7NO2S, is biosynthesized from a substance called cystathionine by a multistep pathway. (a) The first step is a transamination. What is the product? (b) The second step is...
-
The herbicide trifluralin is prepared by the following sequence of reactions. Identify compound A and deduce the structure of trifluralin. CF3 (CH,CH,CHNH Trifluralin HCompound A CH CIFN204) Cl
-
The SEC Form 10-K of Google is reproduced online at www.wiley.com/college/pratt. REQUIRED: Review the Google 2012 SEC Form 10-K, and answer the following questions: a. Compute Googles long-term debt...
-
A former dean of the Kellogg School of Management used to warn faculty not to gloat about the school's #1 ranking in Business Week. Why do you suppose he issued this warning?
-
An objects moment of inertia is 2.0 kg m 2 . Its angular velocity is increasing at the rate of 4.0 rad/s per second. What is the net torque on the object?
-
Working backward, the minimum percentage ownership you need to offer your equity investor to meet, but not exceed, his IRR hurdle rate.
-
When rental cars are sold on the used car market, they are sold for lower prices than cars of the same model and year that were owned by individual owners. Does this price difference reflect adverse...
-
An analyst is evaluating securities in a developing nation where the inflation rate is very high. As a result, the analyst has been warned not to ignore the cross-product between the real rate and...
-
The net income of Foster Furniture, Inc., amounted to $1,920,000 for the current year. a. Compute the amount of earnings per share assuming that the shares of capital stock outstanding throughout the...
-
How could you prepare the following Cyclohexanone by combining a Stork enamine reaction with an intra molecular aldol condensation? (b) (c) (a) CH CH
-
The Knoevenagel reaction is a carbonyl condensation reaction of an ester with an aldehyde or ketone to yield an ?, ??un-saturated product. Show the mechanism of the Knoevenagel reaction of diethyl...
-
How can the staff organize, both in meetings and outside of meetings, to maximize interactions about issues important to the school?
-
Compared to other majornations, the United States spends________ on health care and achieves________ efficiency. A. more; about the same B. about thesame; less C. more; less D. less; less E. less;...
-
Studying other cultures through a humanistic lens allows people to understand how different cultures came about and how and why people behave differently from one place to another (Lombrozo, 2015)....
-
4. Assume that G and T are exogenous, and C is determined by the standard. consumption function, but that investment is now endogenous and responds to income: I = b + bY. Assume c + b < 1. (a)...
-
4. You have decided it's time to buy a house, and you have found the one you want. The price is $500,000, and you will pay 10% in cash and will take a mortgage on the balance. The annual interest...
-
Differentiate. G(x) = (2x+3) (9x+ (x) G'(x)=
-
The Human Relations Model of employee motivation focuses primarily on A. Employee wages B. Employee social relations C. Leadership personality traits D. Leadership behavioral traits
-
The Taylor's series expansion for cosx about x = 0 is given by: where x is in radians. Write a user-defined function that determines cosx using Taylor's series expansion. For function name and...
-
Write the electron configuration for N. Then write the Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
-
The solutions to Solved Problem 8-5 and Solved Problem 8-6 showed only how one enantiomer of the product is formed. For each product, show how an equally probable reaction forms the other enantiomer.
-
Predict the major product(s) for each reaction. Include stereochemistry where appropriate. (a) 1-methylcyclohexene + Cl2 / H2O (b) 2-methylbut-2-ene + Br2/ H2O (c) cis-but-2-ene + Cl2 / H2O (d)...
-
Show how you would accomplish the following synthetic conversions. (a) 3-methylpent-2-ene: 2-chloro-3-methylpentan-3-ol (b) chlorocyclohexane : trans-2-chlorocyclohexanol (c) 1-methylcyclopentanol:...
-
How do external factors such as changing consumer preferences affect the retail industry?"
-
Production costs that are not attached to units that are sold are reported as: Cost of goods sold Selling expenses Administrative costs Inventory
-
Please show workings :) Oxford Company has limited funds available for investment and must ration the funds among four competing projects. Selected information on the four projects follows: Life of...

Study smarter with the SolutionInn App


