Below are two hypothetical compounds. a) Which compound would you expect to hold greater promise as a
Question:
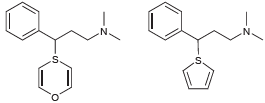
a) Which compound would you expect to hold greater promise as a potential antihistamine? Explain your choice.
b) Do you expect the compound you chose (in part a) to exhibit sedative properties? Explain your reasoning.
Transcribed Image Text:
N. の
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
a The second compound holds greater promise as a potential antihistamine be...View the full answer

Answered By

Muhammed Ajnas
I am a B.tech final year student. My subject is polymer science and engineering. I am a part-time tutor in kerala. I can teach chemistry,physics , biology and mathematics for 8-12 classes
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which compound would you expect to be a stronger acid? Why? CH C-O-H or CH,S -O-H
-
In each of the following pairs, which compound would you expect to have the higher standard molar entropy: (a) C2H2(g) or C2H6(g) (b) CO2(g) or CO(g)? Explain.
-
a. Which compound would you expect to have a higher dipole moment, methyl acetate or butanone? b. Which would you expect to have a higher boiling point? CH3COCH methyl acetate CH,CCH,CH butanone
-
Returning to the data set canadaemplmntdata from Problem 17.4, get a line chart of Accommodation jobs by subsetting by VECTOR = v81682. Problem 17.4 The file canadaemplmntdata contains quarterly...
-
What is intellectual property? What tools do entrepreneurs have to protect their intellectual property?
-
Verify the Divergence Theorem by evaluating as a surface integral and as a triple integral. SS ISJ F. NdS
-
16-3. Describe three common forms of advertising appeals.
-
How are operating expenses (not included in cost of goods sold) handled under the installment-sales method of accounting? What is the justification for such treatment?
-
Ursus, Incorporated, is considering a project that would have a five-year life and would require a $2,400,000 investment in equipment At the end of five years, the project would terminate and the...
-
A statistics professor is in a fantasy golf league with four friends. Each week one of the five people in the league is the winner of that weeks tournament. During the 2010 season, this particular...
-
An electrical motor is used to operate a Carnot refrigerator with an interior temperature of 0.00C. Liquid water at 0.00C is placed into the refrigerator and transformed to ice at 0.00C. If the room...
-
An air conditioner is a refrigerator with the inside of the house acting as the cold reservoir and the outside atmosphere acting as the hot reservoir. Assume that an air conditioner consumes 1.70 ...
-
Evaluate the integral. t csc 2 t dt
-
Identify a public conflict (such as a recent Congressional debate or even a celebrity breakup) that has come to the forefront in the media (or public's attention) in the last thirty days. You have...
-
Performance Management Issues You have been asked to return to your alma mater and speak to current students about performance management issues. To make the most of this experience for yourself and...
-
Analysis of competitor organization of our selected organization Walmart and its competitor Safeway. 1. Complete analysis of competitor organization; addresses all relevant factors and typically uses...
-
Defining Program Objectives of Youth centers Clearly define the objectives of your program or center. What specific outcomes do you hope to achieve? Examples may include promoting physical fitness,...
-
Identify a local or regional organization and analyze how they demonstrate servant leadership in their operations. You will want to review their website, social media, news, and other resources to...
-
In Exercises 3944, an equation of a quadratic function is given. a. Determine, without graphing, whether the function has a minimum value or a maximum value. b. Find the minimum or maximum value and...
-
In the figure, two loudspeakers, separated by a distance of d1 = 2.63 m, are in phase. Assume the amplitudes of the sound from the speakers are approximately the same at the position of a listener,...
-
How would the rate of styrene hydration in H2O/H3O+ differ from that of an isotopically substituted styrene Ph---CH===CD2,? Explain.
-
What product (s) are expected in the ethoxide-promoted - elimination reaction of each of the following compounds? 1-chloro-1 methylcylohexane
-
Predict the products, including their stereochemistry, from the E2 reactions of the following diastereomers of stilbene dibromide with sodium ethoxide in ethanol. Assume that one equivalent of HBr is...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


