Predict the products, including their stereochemistry, from the E2 reactions of the following diastereomers of stilbene dibromide
Question:
Predict the products, including their stereochemistry, from the E2 reactions of the following
diastereomers of stilbene dibromide with sodium ethoxide in ethanol. Assume that one
equivalent of HBr is eliminated in each case.
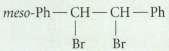
Transcribed Image Text:
meso-Ph -CH- CH Ph Br Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
To conclude we can say that the alkene formed has the E ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
(a) Give the product(s) and their stereochemistry when trans-2-bttene reacts with Hg(OAc), and H2O. (b) What compounds result when the products of part (a) are treated with NaBD4 in aqueous NaOH?...
-
Predict the products from the following reactions. (a) (b) (c) (d) (1) 2 exces) NaoH, Ho (2) H,o KOH 2 HOEIOH KOH H2O/EtOH (1) LDA (1.1 equiv) (3) H2o
-
Explain which of these alkyl chlorides reacts faster with sodium ethoxide in ethanol.
-
(a) Find the Laplace transform of the voltage shown in Fig. 16.52(a). (b) Using that value of vs (t) in the circuit shown in Fig. 16.52(b), find the value of v0 (t). v,(0) 3 V 0 1s al 112
-
Discuss which of the 10 surprising facts about money impacts you most significantly and why.
-
Drum Ltd. makes a single product, using a process involving stamping a circle out from sheet steel, covering it with hide and attaching it to a sidepiece. For 2004 the standard materials costs and...
-
Do Nike's functional strategies support its competitive strategy? Explain.
-
An economist reports that 560 out of a sample of 1,200 middle-income American households actively participate in the stock market. a. Construct the 90% confidence interval for the proportion of...
-
Question 1 ANP Bank started its operation as a commercial and investment bank three decades ago. The environment that the bank operates is highly competitive and volatile in terms of business risk....
-
Huimin Ltd. had the following account balances at December 2021 1 3 25 29 32 30 33 Huimin Ltd. had the following account balances at Decemb Account Name Dividends Prepaid expenses Interest...
-
What product (s) are expected in the ethoxide-promoted - elimination reaction of each of the following compounds? 1-chloro-1 methylcylohexane
-
Draw the structure of the starting material that would undergo azir-elimination give the -E isomer of the alkene product in the E2 reaction of Eq. 9.40.
-
For each of the quality advocates referenced in question one, identify a quality idea\perspective and discuss a corresponding practice in place today at Gold + Williams.
-
Ja-San Company was started on January 1,2007, when the owners invested \($160,000\) cash in the business. During 2007, the company earned cash revenues of \($90,000\) and incurred cash expenses of...
-
Write a program using the programming language of your choice to implement the representation you designed for Review Question 3.3. Have your program solve the problem, and have it show on the screen...
-
All the lenses in Figure P33.98 are surrounded by air. Which of the lenses are converging, and which are diverging? Data from Figure P33.98 A B C D E F )(II)
-
Change the Growth and GrowthDriver classes described in the Improved Accuracy and Efficiency. Using a Step-with-Midpoint Algorithm subsection. Run your modified program with these inputs: For your...
-
For the three-element series circuit in Fig. 9-39, (a) Find the current I; (b) Find the voltage across each impedance and construct the voltage phasor diagram which shows that V 1 + V 2 + V 3 = 100 0...
-
How do securities laws affect the payment structure?
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Predict the NMR spectrum, including approximate chemical shifts, of the following compound. Explain your reasoning. OCH3 CH3O-CH-C-CH-OCH3 CH OCH3
-
(a) Consider entries 1 through 4 of Table 13.1. How does the chemical shift of a proton vary with the electronegativity of the neighboring halogen? (b) Compare entries 2, 5, and 6 of Table 13.1. How...
-
How would you use 13 C NMR spectroscopy to differentiate the two isomers 1-chloropentane and 3-chloropentane?
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


