Predict the NMR spectrum, including approximate chemical shifts, of the following compound. Explain your reasoning. OCH3 CH3O-CH-C-CH-OCH3
Question:
Predict the NMR spectrum, including approximate chemical shifts, of the following compound. Explain your reasoning.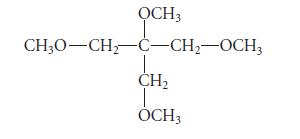
Transcribed Image Text:
OCH3 CH3O-CH₂-C-CH₂-OCH3 CH₂ OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The spectrum will contain three resonances which arise fr...View the full answer

Answered By

Gaurav Soni
Teaching was always an area where I can pursue my passion. I used to teach my friends and junior during my school and college life. After completing my professional qualification (chartered accountancy) and before joining my job, I also joined an organization for teaching and guidance to my juniors. I had also written some articles during my internship which later got published. apart from that, I have also given some presentations on certain amendments/complex issues in various forms.
Linkedin profile link:
https://www.linkedin.com/in/gaurav-soni-38067110a
5.00+
7+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the approximate chemical shifts of the protons in the following compounds. (a) Benzene (b) Cyclohexane (c) CH3-O-CH2CH2CH2Cl (d) CH3CH2-C¡¡C-H (e) (f) (CH3)2CH-O-CH2CH2OH (g) (h)...
-
Predict the chemical shifts for the signals in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) (d) (e)
-
The proton NMR spectrum of 2-pyridone gives the chemical shifts shown. (a) Is 2-pyridone aromatic? (b) Use resonance forms to explain your answer to (a). Also explain why the protons at (7.31and...
-
What was the action you are most proud of, or that is the most meaningful to you? What was the civic issue that was involved in your action? When and how did you first hear about that issue? Why did...
-
How is an NSF check accounted for in the accounting records?
-
In Exercises 117 and 118, as well as many other situations, one has the pdf f(x) of X and wishes to know the pdf of y = h(X). Assume that h( ) is an invertible function, so that y = h(x) can be...
-
Learn the driving forces of globalization. L01
-
Donner Company began its operations in September of the current year. During September, the company paid wages of $23,400. For the last quarter of the year, the taxable wages paid amounted to...
-
Quamina Company manufactures a single product that sells for $205 per unit and whose total variable costs are $164 per unit. The company's annual fixed costs are $553,500. (a) Compute the company's...
-
Propose a structure for a compound with the formula C 7 H 14 with the NMR spectrum shown in Fig. 13.18. Explain in detail how you arrived at your structure. 2400 absorption Lii 8 2100 7 1800 CH14 6...
-
(a) Consider entries 1 through 4 of Table 13.1. How does the chemical shift of a proton vary with the electronegativity of the neighboring halogen? (b) Compare entries 2, 5, and 6 of Table 13.1. How...
-
Information from the records of Powertools Pty Ltd for the year ended 30 June 2025 is given below. Required Calculate the ending work in process inventory on 30 June 2025. Factory overhead, 150% of...
-
Evaluate the following definite integrals as limit of sums: 1. (x (x-x)dx [(2x 2. (2x+5x)dx 3. (2x + +3x+1)dx
-
Evaluate as limit of sums Jr- 3+1dx 1
-
1. 3. Example: Evaluate the following definite integrals as limit of sums: e'dx fedx 2. Je dx 4.
-
Evaluate the following definite integrals as limit of sums: 1. 2 2. 5*x
-
Evaluate the following definite integrals as limit of sums: 1. sin sinxdx 2. 0 /4 cos.xdx 3. sin x dx 2/6
-
Give a rigorous proof that if And Then lim,f(x) = A im g(x) lim [f(x) B
-
Annual dividends of ATTA Corp grew from $0.96 in 2005 to $1.76 in 2017. What was the annual growth rate?
-
Pyridine is a flat, hexagonal molecule with bond angles of 120?. It undergoes substitution rather than addition and generally behaves like benzene. Draw a picture of the ? orbital?s of pyridine to...
-
To be aromatic, a molecule must have 4n + 2? electrons and must have cyclic conjugation. 1, 3, 5, 7, 9-Cyclodecapentaene fulfills one of these criteria but not the other and has resisted all attempts...
-
Draw the five resonance structures of the cyclopentadienyl anion. Are all carbon carbon bonds equivalent? How many absorption lines would you expect to see in the 1H NMR and 13C NMR spectra of the...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


