(a) Consider entries 1 through 4 of Table 13.1. How does the chemical shift of a proton...
Question:
(a) Consider entries 1 through 4 of Table 13.1. How does the chemical shift of a proton vary with the electronegativity of the neighboring halogen?
(b) Compare entries 2, 5, and 6 of Table 13.1. How does chemical shift vary with the number of neighboring halogens?
(c) Compare entries 6 and 7. How is the chemical shift of a proton affected by its distance from an electronegative group?
(d) Explain why (CH3)4Si absorbs at lower chemical shift than the other molecules in the table. Can you think of a molecule with protons that would have a smaller chemical shift than TMS (that is, a negative d value)?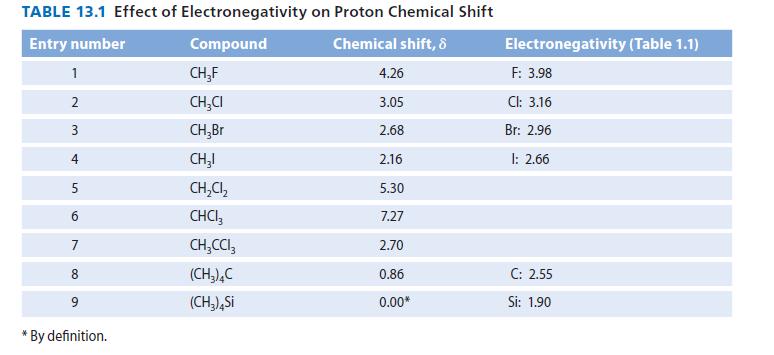
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





