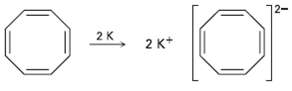
Cyclooctatetraene readily reacts with potassium metal to form the stable Cyclooctatetraene dianion, C 8 H 8 2
Question:
Cyclooctatetraene readily reacts with potassium metal to form the stable Cyclooctatetraene dianion, C8H82?1. Why do you suppose this reaction occurs so easily? What geometry do you expect for the Cyclooctatetraene dianion?
Transcribed Image Text:
2K 2 K* 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
When Cyclooctatetraene accepts two ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Why do you suppose that forensic DNA analysis relies principally on short tandem repeats (repeat polymorphisms), rather than single- nucleotide polymorphisms, such as that described on page 669 and...
-
Why do you suppose that large, well-known companies such as Apple, Starbucks, and Facebook prefer to have their shares traded on the NASDAQ rather than on one of the major listed exchanges, such as...
-
Why do you suppose that m x n machine-scheduling algorithms are not widely used in practice? Should optimal rules be more widely used?
-
Fewer than 20% of M&M candies are green. The hypothesis test results in a P-value of 0.0721. Assume a significance level of = 0.05 a. State a conclusion about the null hypothesis. b. Without using...
-
Who is affected by a hospital's decision not to hire smokers? Discuss whether this decision achieves the greatest good for the greatest number of individuals.
-
Midwest Inc. is a medium-size company that has been in business for 20 years. The industry has become very competitive in the last few years, and Midwest has decided that it must grow if it is going...
-
Discuss your favorite theme park with your class. Explain why it is your favorite. LO.1
-
Apache, a U.S. corporation, owns 80% of the stock in Burrito, incorporated in Country Y. Burrito reports the following results for the current year: ______________________________________ Gross...
-
7:09 docs.google.com/forms/d/ 94* Cotisaster the following users 3/5/7/19/675 and the Modificatiu putin siven by y - 10x15 Then the writhmetic mean hier tiedication ( ) ) O 400 0410 0445 O 335
-
On April 1, Cyclone Company purchases a trencher for $308,000. The machine is expected to last five years and have a salvage value of $54,000. Compute depreciation expense at December 31 for both the...
-
Draw the five resonance structures of the cyclopentadienyl anion. Are all carbon carbon bonds equivalent? How many absorption lines would you expect to see in the 1H NMR and 13C NMR spectra of the...
-
Draw an orbital picture of furan to show how the molecule isaromatic. Furan :O:
-
Determine the autocorrelation function for the rectangular wave shown in Figure P13.2. A F -2T -15T -T -7T T T 9T 2T 17T t 8 8 8 8 8 FIGURE P13.2
-
Analysis of workforce data, performance, and engagement. Datasets: Employees Table Column Name Data Type Description employee_id Integer Unique identifier for each employee department_id Integer...
-
Discuss your observations of the Data Wrangling process. Does this exercise highlight why data wrangling and preparation can take up 60-70% of the total data analysis process? it does. How do i say...
-
Examine potential implications od regulations, legislation and standards upon decision making in a hospitality organisation, providing specific examples
-
54. .. A baton twirler in a marching band complains that her baton is defective (Figure 9-48). The manufacturer specifies that the baton should have an overall length of L = 60.0cm and a total mass...
-
New United Motor Manufacturing, Inc. was an American automobile manufacturing company in Fremont, California , jointly owned by General Motors and Toyota that opened in 1 9 8 4 and closed in 2 0 1 0...
-
understand the need for bank reconciliations and prepare such reconciliations.
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
For the reaction, A(g) 2 B( g), K c = 4.0. A reaction mixture at equilibrium contains [A] = 1.0 M. What is the concentration of B in the reaction mixture? (a) 0.50 M (b) 1.0 M (c) 2.0 M (d) 4.0 M
-
(a) Show that D-glucose, D-mannose, and D-fructose all give the same osazone. Show the structure and stereochemistry of this osazone. (b) D-Talose is an aldohexose that gives the same osazone as...
-
Show that Ruff degradation of D-mannose gives the same aldopentose (D-arabinose) as does D-glucose.
-
D-Lyxose is formed by Ruff degradation of galactose. Give the structure of D-lyxose. Ruff degradation of D-lyxose gives D-threose. Give the structure of D-threose.
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


