To be aromatic, a molecule must have 4n + 2? electrons and must have cyclic conjugation. 1,
Question:
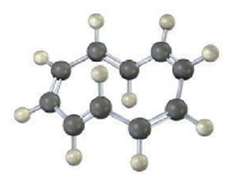
To be aromatic, a molecule must have 4n + 2? electrons and must have cyclic conjugation. 1, 3, 5, 7, 9-Cyclodecapentaene fulfills one of these criteria but not the other and has resisted all attempts at synthesis. Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (18 reviews)
H H Cyclodecapentaene has 4n 2x electrons n2 ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The allene molecule has the following Lewis structure: Must all four hydrogen atoms lie in the same plane? If not, what is the spatial relationship among them? Why? C=C=C
-
Explain why attempts at phagocytosis are not always successful; cite factors that increase the likelihood of success.
-
Explain the symmetry criteria that allow a molecule to be polar?
-
On December 31, 2021, there is a batch of commodities sold under FOB destination conditions in the shipping area, and this batch of commodities is not included in the inventory count middle. There is...
-
Do the results of this survey indicate that U. S. Companies should spend up to $50,000 to select an employee for every vacant position? Why or why not?
-
What is the magnetic field at the position of the dot in FIGURE EX29.6? Give your answer as a vector. y 1.0 cm 2.0 x 10" m/s 1.0 cm Proton FIGURE EX29.6
-
Why did Milton Hershey open the leisure park? LO.1
-
Accounting and financial reporting for state and local governments use, in different places, either the economic resources measurement focus and the accrual basis of accounting or the current...
-
Problem 14-02 (Part Level Submission) Coronado Co. is building a new hockey arena at a cost of $2,360,000. It received a downpayment of $500,000 from local businesses to support the project, and now...
-
Your client, Jon's Auto Parts Ltd., a Canadian-controlled private corporation, has prepared its own financial statements for the year ended December 31, 2019. The financial statements are not...
-
Pyridine is a flat, hexagonal molecule with bond angles of 120?. It undergoes substitution rather than addition and generally behaves like benzene. Draw a picture of the ? orbital?s of pyridine to...
-
Draw the five resonance structures of the cyclopentadienyl anion. Are all carbon carbon bonds equivalent? How many absorption lines would you expect to see in the 1H NMR and 13C NMR spectra of the...
-
Explain the importance of internal control to a business and the significance of the Sarbanes-Oxley Act of 2002.
-
Explain in simple terms the concept of: "Technology Structures and Social Boundaries"
-
1- According to the Six Steps in Strategic Planning find out the Lidl and Mercadona strategic plan 2021-2022 in Spain. Highlight the major differences and similarities between them. 2- Make a picture...
-
Your writing must present an introduction, development and conclusion. At the end of your work include the APA references. case 1: program for the agency that provides services to the government...
-
7. (8 points) In the following VHDL process, if input A changes at time 20nS and no other inputs change after that time, at what time will all the output signals be guaranteed to have assumed their...
-
Q1. Tenure analysis: Table: employee Column Name Data Type Description employee_id Integer Unique identifier for each employee department Varchar The department of the employee job_level Varchar The...
-
understand the problems caused by having numerous personal accounts (in a manual system) and the consequent need for control accounts;
-
If the joint cost function for two products is C(x, y) = xy2 + 1 dollars (a) Find the marginal cost (function) with respect to x. (b) Find the marginal cost with respect to y.
-
Does the value of the equilibrium constant depend on the initial concentrations of the reactants and products? Do the equilibrium concentrations of the reactants and products depend on their initial...
-
Show the products that result from hydrolysis of amygdalin in dilute acid. Can you suggest why amygdalin might be toxic to tumor (and possibly other) cells?
-
Treatment of either anomer of fructose with excess ethanol in the presence of a trace of HCl gives a mixture of the and anomers of ethyl-D-fructofuranoside. Draw the starting materials, reagents,...
-
Propose a mechanism for methylation of any one of the hydroxyl groups of methyl -D-glucopyranoside, using NaOH and dimethyl sulfate.
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


