Compare the structures of a carbocation and a carbanion: In one of these ions, the central carbon
Question:
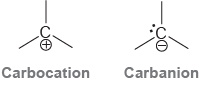
In one of these ions, the central carbon atom is trigonal planar; in the other it is trigonal pyramidal. Assign the correct geometry to each ion
Transcribed Image Text:
Carbanion Carbocation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
The carbon atom of the carbocation has t...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compare the structures of a synchondrosis and a symphysis.
-
Compare the structures of lanosterol and cholesterol, and catalog the changes needed for the transformation.
-
Compare the structures of HNO2 and H2CO3. Which would you expect to be the stronger acid? Explain your choice.
-
In Problems 1318, express the graph shown in blue using interval notation. Also express each as an inequality involving x. -1 0 1 2 3
-
Write a paper comparing and contrasting the five categories of drug treatment.
-
Use a calculator to evaluate the given expressions. tan 1 (2.8229)
-
6. Supposing the effective quarterly interest rate is 1.5%, what are the per-barrel swap prices for 4-quarter and 8-quarter oil swaps? (Use oil forward prices in Table 9.) What is the total cost of...
-
Reza Lang is the bookkeeper for Taylor Company. Reza has been trying to get the balance sheet of Taylor Company to balance. Taylors balance sheet is shown below. Instructions Prepare a correct...
-
Rock Bottom Gold Company recently repurchased 7.14 million shares of its common stock for $43 per share. The intent of the repurchase was to increase earnings per share to be more in line with...
-
Write a program that will take as input two Web page URLs and find a path of links from one to the other. What is an appropriate search strategy is bidirectional search a good idea? Could a search...
-
Predict the geometry for all atoms except hydrogen in the compounds below: a. b. c. H || 0-H .C. C-H H-C H-C C-H H H H-C-H H
-
Treatment of (2R, 3R)-3-methyl-2-pentanol with H 3 O + affords a compound with no chirality centers. Predict the product of this reaction and draw the mechanism of its formation. Use your mechanism...
-
When does a company have a deficit in retained earnings?
-
Why the sudden increase in income before taxes in 2021? 8. Why were the operating assets the highest in 2019? 9. Why are the short-term loans the highest in 2020? 10. Why are the other long-term...
-
Mercy wants to make sure that she will be able to provide for her daughter's college and plans to open a savings account with a bank that is ready to pay interest as shown below per year compounded...
-
Question 1. For a firm that uses portfolio management, please give a real or hypothetical example of how the CEO's personal bases for power help organizational performance. Question 2. Give a real...
-
Make a schedule that you would use that effectively illustrates working with paraprofessionals that includes collaboration time. Use the examples provided in the following resources to guide your...
-
How does the integration of technology and automation influence employee motivation and job satisfaction within modern organizational contexts ?
-
Write the radical expression using positive exponents. AAXA
-
On August 31, 2012, the balances of the accounts appearing in the ledger of Wood Interiors Company, a furniture wholesaler, are as follows:Prepare the August 31, 2012, closing entries for Wood...
-
Isopropyl alcohol is produced commercially by the hydration of propene. Show the mechanistic steps of this process. If you do not know the structure of isopropyl alcohol, try to deduce it by analogy...
-
Give the structures and the IUPAC substitutive names of the isomeric alkenes with the molecular formula CuH,, containing four carbons in their principal chain.
-
Give a structure for each of the following compounds. (a) 3-methyl- 1-octene (b) Isoprene
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


