Compound A has molecular formula C10H10O and exhibits a strong signal at 1720 cm1 in its IR
Question:
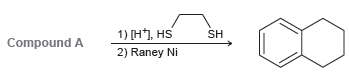
Transcribed Image Text:
1) [H*), HS 2) Raney Ni SH Compound A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
because th...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compound A has molecular formula C 5 H 10 . Hydroboration- oxidation of compound A produces 2-methylbutan-1-ol. Draw the structure of compound A: Compound A (C,H10) 1) BH, THF 2) H202, NaOH
-
Compound A has molecular formula C 8 H 14 O 2 . Upon treatment with catalytic acid, compound. A is converted into the cyclic hemiacetal. Identify the structure of compound A. , Compound A [H*]
-
Treating 1,2-cyclohexanediol with concentrated sulfuric acid yields a product with molecular formula C 6 H 10 O. An IR spectrum of the product exhibits a strong signal at 1720 cm -1 . Identify the...
-
Find the deflection y(x) of a cantilever beam embedded at its left end and free at its right end when the load is as given in Example 10.
-
On September 11, 2014, the Hafar Corporation has unrestricted Retained Earnings of $6,000,000. Appropriated Retained Earnings of $4,000,000. Cash of $7,500,000. and Accounts Payable of $500,000. What...
-
AZCN recommends Microsoft Lens or Adobe Scan; download one of these to yo phone via your phone's app store 2. Place the document you want to scan on a flat, well-lit surface. Make sure the document...
-
Measuring pain. The Department of Veterans Affairs offers medical care to 5.5 million patients. It wants doctors and nurses to treat pain as a fifth vital sign, to be recorded along with blood...
-
Charter One Bank owned a fifteen-story commercial building. A fire inspector told Charter that the building's drinking water and fire suppression systems were linked. Without disclosing this...
-
Please give all ( a ,b ,c, d , e ) requirements, otherwise, skip questions. The current price of Gold plc is 154p. It has a policy of paying out 45 per cent of earnings in dividends each year. The...
-
The ages of 20 dogs in a pet shelter are shown. Construct a frequency distribution using 7 classes. 3 6. 4 4 9. 4 3 4 9.
-
Using any compounds of your choosing, identify a method for preparing each of the following compounds. Your only limitation is that the compounds you use can have no more than two carbon atoms. For...
-
Provide a systematic (IUPAC) name for each of the following compounds: (a) (b) (c) (d) H.
-
The population (in millions) of Americans under the age of 18 for years from 2016 and projected through 2050 is shown in the table. Construct a bar graph of the population for the given time...
-
Shire Company's predetermined overhead rate is based on direct labor cost. Management estimates the company will incur $649,000 of overhead costs and $590,000 of direct labor cost for the period....
-
You plan to live 25 years after you retire. You want to withdraw $100,000 each year for 25 years. Your first withdrawal will take place the day after you retire. What is the four annuity formulas...
-
Harwood Company's quality cost report is to be based on the following data: 2021 2022 Depreciation of test equipment $94,000 $95,000 Audits of the effectiveness of the quality system $54,000 $51,000...
-
Cash contribution of 4,000 to the Accounting Society (a charity) Purchase of art object at an Accounting Society Charitable event for $1,200 (FMV $800) Donation of 3-year-old clothing (basis 800; FMV...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
A chemist needs 12 L of a 40% alcohol solution. She must mix a 20% solution and a 50% solution. How many liters of each will b required to obtain what she needs? Liters of Solution Percent (as a...
-
Selected condensed data taken from a recent statement of financial position of Morino Ltd. are as follows. MORINO LTD. Statement of Financial Position (partial) Other current assets...
-
Write the structure(s) of the simplest alkane(s), i.e., one(s) with the fewest number of carbon atoms, wherein each possesses primary, secondary, tertiary, and quaternary carbon atoms. (A quaternary...
-
Ignoring compounds with double bonds, write structural formulas and give names for all of the isomers with the formula C5H10.
-
Write structures for the following bicyclic alkanes: (a) Bicyclo [1.1.0] butane (b) Bicyclo [2.1.0] pentane (c) 2-Chlorobicyclo [3.2.0] heptane (d) 7-Methylbicyclo [2.2.1] heptane
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


