Compound A has molecular formula C 5 H 10 . Hydroboration- oxidation of compound A produces 2-methylbutan-1-ol.
Question:
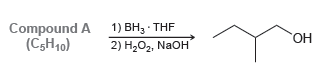
Compound A (C,H10) 1) BH, THF 2) H202, NaOH ОН
Step by Step Answer:
1 BH...View the full answer



Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Sciences questions
-
Compound A has molecular formula C 5 H 10 . Hydroboration-oxidation of compound A produces a pair of enantiomers, compounds B and C. When treated with HBr, compound A is converted into compound D,...
-
Compound A has molecular formula C 5 H 10 . Hydroboration-oxidation of compound A produces an alcohol with no chirality centers. Draw two possible structures for compound A.
-
Compound A has molecular formula C5H12, and monobromination of compound A produces only compound B. When compound B is treated with a strong base, a mixture is obtained containing compound C and...
-
Instructions: Read the footnotes included in the financial statements for H & B Bakery, then answer the following questions. *The exact requirement of this question, is to read the statements below...
-
Why do some companies prefer the use of economic profit over return on investment in decisionmaking? A. The data needed to calculate return on investment are not always available. B. The calculations...
-
In Exercises determine whether the statement is true or false. If it is false, explain why or give an example that shows it is false. If u and v have the same magnitude but opposite directions, then...
-
2 What kind of compromise solution would have been acceptable to both teams?
-
Joanne is a seventy-five-year-old widow who survives on her husbands small pension. Joanne has become increasingly forgetful, and her family worries that she may have Alzheimers disease (a brain...
-
Georgia Orchards produced a good crop of peaches this year. After preparing the following income statement, the company is concerned about the net loss on its No. 3 peaches. GEORGIA ORCHARDS Income...
-
A thick steel sheet of area 400 cm2 is exposed to air near the ocean. After a one-year period it was found to experience a weight loss of 375 g due to corrosion. To what rate of corrosion, in both...
-
Below are several examples of hydroboration-oxidation. In each case, consider the expected regioselectivity, and then draw the product: a. b. c. 1) , THF 2) H,O2, NaOH 1) BH3 THF 2) H2O2, NaOH
-
Explain how the ideal gas law can be deduced for the measurements shown in Figures 1.5 and 1.8. Figure 1.5 Figure 1.8 0.1 L 2.5 2.0 1.5 0.2 L 1.0 0.3 L 0.4 L 0.5 L 0.6 L '0.5 -200 100 0 100 200 300...
-
Hydrogen peroxide, H 2 O 2 , reacts with sulfur trioxide to form peroxomonosulfuric acid, H 2 SO 5 , in a Lewis acidbase reaction. (a) Write the chemical equation for the reaction. (b) Draw the Lewis...
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
In Exercises 9 through 16, find all vertical and horizontal asymptotes of the graph of the given function. h(x) || 1 1 1 x-1 X X
-
Given that all the choices are true, which one concludes the paragraph with a precise and detailed description that relates to the main topic of the essay? A. NO CHANGE B. Decades, X-ray C. Decades...
-
Predict the product of the following Diels-Alderreaction: - C=C
-
Which of the following alkenes would you expect to be good Diels-Alder dienophile?s? (b) (a) H2C=CHCI %3H2H2C (d) (c) (e)
-
Which of the following dienes have an s-cis conformation, and which have an s-trans conformation? Of the s-trans dienes, which can readily rotate tos-cis? (a) (c) (b)
-
Nitin is paid a base salary of $200 per week and commission at the rate of 3% for sales over $5000, 4% if his sales are over $8000, and 5% if sales are over $15,000. How much will Nitin earn in a...
-
Safa is paid a base salary of $1500 per month and a commission of 6% on all sales over $75,000. Last month, Safa's gross salary was $4440. What were her sales for the month? a$149,000 b$124,000...
-
Your regular hourly rate of pay is $15.86, and you are paid double time for all work on weekends and for any time over forty hours per week (Monday to Friday). Calculate your gross earnings for a...

Study smarter with the SolutionInn App


