Which of the following alkenes would you expect to be good Diels-Alder dienophile?s? (b) (a) H2C=CHCI %3H2H2C
Question:
Which of the following alkenes would you expect to be good Diels-Alder dienophile?s?
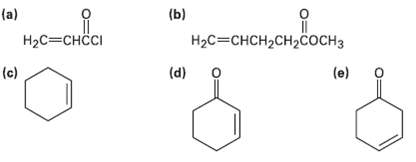
Transcribed Image Text:
(b) (a) H2C=CHCI Нас%3снсH2сH2Cоснз (d) (c) (e)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Strategy Good dienophiles have an electronwithdrawing group conjugated with a double bond Good di...View the full answer

Answered By

Rohit anand
i have done btech from nit,hamirpur..i teach physics. i am teaching physics from last 2 year ..
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of the following molecules would you expect to be aromatic? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) N+
-
Which of the following processes would you expect to be under control, and which would you expect not to be under control? Explain briefly why or why not. (a) Daily sales at each checkout line in a...
-
Which of the following nuclides would you expect to be radioactive: tungsten-184, polonium-206? Justify your choices. Ni, Cu, Ag, 47 108 Ag.
-
What is the square root of 3 to the square root of 2 power times the square root of 3 to the negative square root of 2 power?
-
How might a persistent global credit crisis affect the scale and scope of modern firms?
-
Jeffery Pickell was the sole proprietor of Kaleidoscope Books in Ann Arbor, Michigan. While Martin Hermans daughter was a student at the University of Michigan, he became a customer of the store. At...
-
Elaborate on the wheel communication network.
-
One day, Leslie prepared a new snack to serve at preschool: celery stuffed with ricotta cheese and pineapple. The first time she served it, few children touched it. How can Leslie encourage her...
-
Required information [The following information applies to the questions displayed below.] Reba Dixon is a fifth-grade school teacher who earned a salary of $38,000 in 2021. She is 45 years old and...
-
Three-Month Project NOTE! Templates needed Ampersand, Inc., is a small business that operates in Somerset, VT The company is located at 732 Appalachian Way, Somerset, VT 05363. Its federal Employer...
-
Predict the product of the following Diels-Alderreaction: - C=C
-
Which of the following dienes have an s-cis conformation, and which have an s-trans conformation? Of the s-trans dienes, which can readily rotate tos-cis? (a) (c) (b)
-
From the information in the first section of the case study, how would you characterize YSNs organizational culture? Do you have enough information to have a sense of the deepest level of its...
-
Mail - Jame Mail - Jame x a Amazon.co x a Amazon.co. X https://gpt x _ Calendar - - C * gptc.blackboard.com/webapps/blackboard/content/contentWrapper.jsp?content_id=_1846554 GEORGIA PIEDMONT...
-
Prepare a partnership return and the appropriate K-1s for W & M Partnership. William Winston (SSN: 226-00-4265) lives at 53 Mantis Road, Your City, Your State - Your Zip. He operates Lovely Lady...
-
A new store is opening in Rock Spring, with 175,000 available square feet. Each department must have at least 17,000 square feet and no department can have more than 24% of the total retail floor...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Stockholders of Sarasota Company, Riverbed Company, and Pronghorn Company are considering alternative arrangements for a business combination. Balance sheets and the fair values of each company's...
-
Torsten Pieper is an experienced qualitative researcher with Mayfair Research, Ltd. His company was contracted to conduct a series of twenty employee focus groups in five countries. Focus-group...
-
You are planning to purchase your first home five years from today. The required down payment will be $50,000. You currently have $20,000. but you plan to contribute $500 each quarter to a special...
-
What are colligative properties?
-
Draw just the bonding -MO's for the cycloheptatrienyl cation. Draw the energy diagram to show the relative energies of all the MO's, and show which orbitals the electrons would occupy in the ground...
-
The proton NMR chemical shifts of the hydrogens in pyridine are shown. These are typical aromatic chemical shifts, except that the ortho protons (on the carbons bonded to nitrogen) are deshielded to...
-
Does the MO energy diagram of cyclooctatetraene (Figure 16-8) appear to be a particularly stable or unstable configuration? Explain. In Figure 16.8 nonbonding line cyclobutadiene cyclooctatetraene...
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....
-
What is the definition of substantially appreciated inventory? A. Inventory with a FMV greater than its basis B. Inventory and unrealized receivables with a FMV greater than their basis C. Inventory...
-
Case Products manufactures two models of DVD storage cases: regular and deluxe. Presented is standard cost information for each model: Cost Components Regular Deluxe Direct materials Lumber 2 board...

Study smarter with the SolutionInn App


