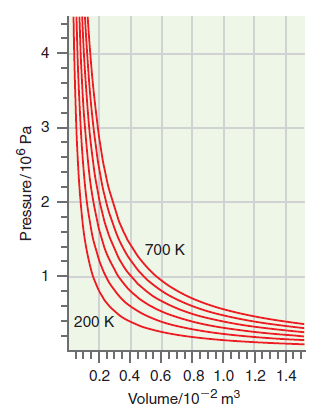
Explain how the ideal gas law can be deduced for the measurements shown in Figures 1.5 and
Question:
Figure 1.5
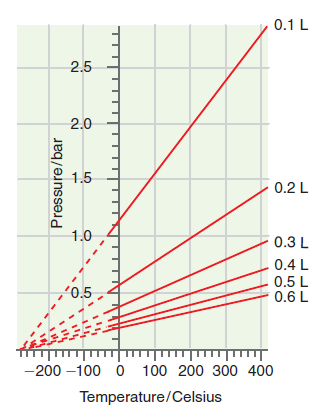
Figure 1.8
Transcribed Image Text:
0.1 L 2.5 2.0 1.5 0.2 L 1.0 0.3 L 0.4 L 0.5 L 0.6 L '0.5 тп -200 –100 0 100 200 300 400 Temperature/Celsius Pressure/bar 700 K 200 K Tпт 0.2 0.4 0.6 0.8 1.0 1.2 1.4 Volume/10-2 m3 3. 2. Pressure/106 Pa
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
Figure 15 shows that at constant volume P increases ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Use the ideal gas law to show that, for an ideal gas at constant pressure, the coefficient of volume expansion is equal to = 1/T, where T is the temperature in kelvins. Compare to Table 13-1 for...
-
Pressure of an ideal gas. It is desired to get the pressure exerted by an ideal gas on a wall by accounting for the rate of momentum transfer from the molecules to the wall.? (a) When a molecule...
-
Adiabatic frictionless processes in an ideal gas (a) A gas that obeys the ideal gas law may deviate appreciably from C p = constant. Hence rework Example 11.4-6 using a molar heat capacity expression...
-
CPA firm brings in a yoga instructor during the tax busy season to help relieve stress of the employees. Which is true about the CPA firm's ability to take a deduction for the yoga instructor's...
-
1) What is HiTech's pool rate for the material-handling activity closest to? 2) What is HiTech's pool rate for the automated machinery activity closest to? 3) What is HiTech's pool rate for the...
-
In Exercises find the distance between the point and the line given by the set of parametric equations. (1,5,-2); x = 4t2, y = 3, z = -t + 1
-
Cross-cultural meetings often have periods of inactivity where nothing seems to be achieved, or where important decisions are made without proper discussion. One reason is that participants from...
-
1. Identify some of the problems likely to occur in a boundaryless organization such as Newskool Grooves. What are the advantages of boundaryless organizations? 2. Consider some of the cultural...
-
When two or more firm merge then the process is known as A . Amalgamation B . Merge C . Reconstruction D . None of the above Skip else i will downvote 1 0 times.
-
Payments of $500 originally scheduled to be paid today and $300 originally scheduled to be paid three months from today are to be replaced with a single payment six months from today. Calculate the...
-
Compound A has molecular formula C 5 H 10 . Hydroboration- oxidation of compound A produces 2-methylbutan-1-ol. Draw the structure of compound A: Compound A (C,H10) 1) BH, THF 2) H202, NaOH
-
A bowling ball (a) Rolls across a table (b) Falls on the floor. Is the work associated with each part of this process positive, negative, or zero?
-
Roney issues $120,000 of 6%, 15-year bonds dated January 1, 2011, that pay interest semiannually on June 30 and December 31. They are issued at $99,247, and their market rate is 8% at the issue date....
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
In Exercises 13 through 16, the derivative f'(x) of a function is given. Use this information to classify each critical number of f(x) as a relative maximum, a relative minimum, or neither. f'(x) =...
-
What are the principal alloying elements in SAE 4340 steel?
-
At 18C the total volume V of a solution formed from MgS04 and 1.000 kg of water fits the expression v = 1001.21 + 34.69(x - 0.070)2, where v = V/cm3 and x = blb-1. Calculate the partial molar volumes...
-
The following table gives the mole fraction of methylbenzene (A) in liquid and gaseous mixtures with butanone at equilibrium at 303.15 K and the total pressure p. Take the vapour to be perfect and...
-
Use the Gibbs-Duhem equation to show that the partial molar volume (or any partial molar property) of a component B can be obtained if the partial molar volume (or other property) of A is known for...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


