The following table gives the mole fraction of methylbenzene (A) in liquid and gaseous mixtures with butanone
Question:
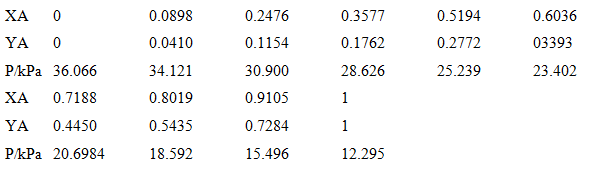
The following table gives the mole fraction of methylbenzene (A) in liquid and gaseous mixtures with butanone at equilibrium at 303.15 K and the total pressure p. Take the vapour to be perfect and calculate the partial pressures of the two components. Plot them against their respective mole fractions in the liquid mixture and find the Henry's law constants for the two components.
Transcribed Image Text:
0.2476 0.3577 0.1762 0.5194 0.6036 0.0898 XA 0.1154 0.0410 0.2772 03393 YA PkPa 36.066 25.239 23.402 28.626 34.121 30.900 0.9105 0.7188 XA 0.8019 0.5435 0.7284 0.4450 YA 12.295 P/kPa 20.6984 15.496 18.592
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
PA YAP and PB BP Daltons law Hence draw up the following table P...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
The following table gives selective data on nominal exchange rates, price levels, and real exchange rates for Country A and several other countries. Country A uses the dollar (A$) as its currency....
-
The following table gives the total points scored by each of the top 16 National Basketball Association (NBA) scorers during the 201011 regular seasons a. Calculate the mean and median. Do these data...
-
The following table gives the top five television shows, as determined by the Nielsen Ratings for the week ending October 19, 2008. Identify the type of data provided by the information in each...
-
int Temp [10] = { 22, 30, 40,28,20,60}; For the above code, answer the following questions i) Find the size of Temp array. ii) How many memory locations are present in Temp array ? iii) Find the...
-
Explain how the cerebellum is physically connected to the brain stem.
-
The Glass Menagerie makes small pressed resin ducks and ducklings. For every duck sold, the company sells five ducklings. The following information is available about the companys selling prices and...
-
To plan the flow of materials through manufacturing, what four things must production activity control know? Where will information on each be obtained? LO.1
-
Define the concept of scalability. Explain why it might be a good idea for owners of small businessesand managers in larger businessesto understand this concept.
-
The Gilster Company, a machine tooling firm, has several plants. One plant, located in St . Cloud, Minnesota, uses a job order costing system for its batch production processes. The St . Cloud plant...
-
On December 31, 2021, when its Allowance for Doubtful Accounts had a zero balance, Ling Co. estimated that 2% of its net accounts receivable of $450,000 will become uncollectible and records the...
-
At 18C the total volume V of a solution formed from MgS04 and 1.000 kg of water fits the expression v = 1001.21 + 34.69(x - 0.070)2, where v = V/cm3 and x = blb-1. Calculate the partial molar volumes...
-
Use the Gibbs-Duhem equation to show that the partial molar volume (or any partial molar property) of a component B can be obtained if the partial molar volume (or other property) of A is known for...
-
Yazaki Corporation and DENSO Corporation are both Japanese companies that supply electrical components to manufacturers of automobiles. Following an investigation by the U.S. Department of Justice in...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
Based on the dangling-else discussion in Exercise 3.27, modify the following code to produce the output shown. Use proper indentation techniques. You must not make any additional changes other than...
-
Consider the random process \(U(t)=A\), where \(A\) is a random variable uniformly distributed on \((-1,1)\). (a) Sketch some sample functions of this process. (b) Find the time autocorrelation...
-
Explain the difference between the one-tailed and two-tailed versions of the Wilcoxon rank sum test for independent random samples.
-
The rate at which the temperature of an object changes is proportional to the difference between its own temperature and the temperature of the surrounding medium. Express this rate as a function of...
-
What are techniques for reducing the risk of drop off, the distortion of a message being sent from top management to subordinates?
-
Calculate the magnitude and direction of the dipole moment of the following arrangement of charges in the xy-plane: 3e at (0,0), e at (0.32 nm, 0), and 2e at an angle of 20 from the x-axis and a...
-
An H 2 O molecule is aligned by an external electric field of strength 1.0 kV m 1 and an Ar atom ( = 1.66 10 24 cm 3 ) is brought up slowly from one side. At what separation is it energetically...
-
The electric dipole moment of toluene (methylbenzene) is 0.4 D. Estimate the dipole moments of the three xylenes (dimethylbenzene). Which answer can you be sure about?
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...
-
Only need help on 4B and 5. Exercise 9-21 Breakeven Planning; Profit Planning (LO 9-2, 9-3] Connelly Inc., a manufacturer of quality electric ice cream makers, has experienced a steady growth in...

Study smarter with the SolutionInn App


