Consider the following reaction: a) How would the rate be affected if the concentration of tertbutyl bromide
Question:
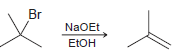
a) How would the rate be affected if the concentration of tertbutyl bromide is doubled?
b) How would the rate be affected if the concentration of sodium ethoxide is doubled?
Transcribed Image Text:
Br NaOEt ETOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a The rate of an E2 process is dependent on the concentrations of the s...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction at some temperature: H2O(g) + CO(g) H2(g) + CO2(g) K = 2.0 Some molecules of H2O and CO are placed in a 1.0- L container as shown below. When equilibrium is reached,...
-
Consider the following reaction at equilibrium: From the data shown here, calculate the equilibrium constant (both KP and Kc) at each temperature. Is the reaction endothermic or exothermic?...
-
Consider the following reaction: CH3X + Y CH3Y + X At 25oC the following two experiments were run, yielding the following data: Experiment 1: [Y]0 = 3.0 M Experiment 2: [Y] 0 = 4.5 M Experiments were...
-
Villaverde Company insures the life of its president for P8,000,000, the corporation being the beneficiary of an ordinary life policy. The premium is P200,000. The policy is dated January 1, 2010....
-
Company to be analyzed: Delta Airlines, Inc, and report structure and requirements are: a. An analysis overview which includes background information of the company and the industry. This should...
-
Evaluate the given determinants. -20 110 -70-80
-
What is gross operating income?
-
Although the equity method is a generally accepted accounting principle (GAAP), recognition of equity income has been criticized. What theoretical problems can opponents of the equity method...
-
ABC Corporation uses customers served as its measure of activity. During February, the company budgeted for 3 7 , 0 0 0 customers, but actually served 2 7 , 0 0 0 customers. The company uses the...
-
Lucy Group moves to fulfil its new strategy to increase the number of its subsidiaries globally. Lucy Group is an Australian Business Developer that assists companies to develop their markets. In the...
-
In the algebraic version of prospect theory, the variable x represents gains and losses. A positive value for x is a gain, a negative value for x is a loss, and a zero value for x represents...
-
Ted has always had difficulty saving money. So on June 1 st , Ted enrolls in a Christmas savings program at his local bank and deposits $750. That money is totally locked away until December 1 st so...
-
Determine which of the following limits exist. Compute the limits that exist. lim 6x + 3x x-6 -)(x-4) (x - 4) X
-
Brian is considering increasing the length of the cryptographic keys used by his organization. If he adds 8 bits to the encryption key, how many more possible keys will be added to the key space for...
-
Business law SECHON A [100 Marks] Read the scenario below then answer the questions that follow. Contracts are of critical importance especially in daily commercial and business transactions....
-
You may assume that the production costs to the winery are the same for each of the possible wines, despite the differences in volumes with some of the possible wines. Thus maximizing revenue will be...
-
You encounter a split system that uses R-22 refrigerant and observe the following refrigeration parameters from the unit's control display. The unit is operating in cooling mode. Suction pressure:...
-
A refrigerant at -20C is flowing through a 4" schedule 40 carbon steel pipe (inner diameter 102 mm, outer diameter 114 mm); the heat transfer coefficient for the refrigerant is 2500 W/m/K. It is...
-
Factor by grouping. p 2 - 4zq + pq - 4pz
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase. ", , (b) . (a) CHCCH-CH2CH2CH3 CH2H2CCgHs o2Et NO2 (d)...
-
The so-called Wieland?Miescher ketone is a valuable starting material used in the synthesis of steroid hormones. How might you prepare it from 1, 3-cyclohexanedione? Wieland-Miescher ketone
-
The following reactions are unlikely to provide the indicated product in high yield. What is wrong witheach? . , (a) Na* "OEt CHH2CH CHCH CHCH Ethanol (b) .oon CH2CH2CCH3 Na* "OEt + H3 Ethanol "H...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...

Study smarter with the SolutionInn App


