How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic
Question:
How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase.
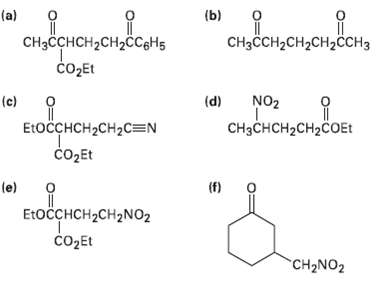
Transcribed Image Text:
"ндо, Понененает, (b) Дененене. (a) CHзCCH-CH2CH2CСH3 CнзсснсH2сH2CCgHs čo2Et NO2 (d) (c) П E:OCCHCH2CH2C=N CHзснсH2сH2cOEt СоДEt (П) (e) EtoсснCH2CH2NO2 COДEL CH2NO2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Michael reactions occur between stabilized enolate anions and ounsaturated carbonyl compounds Learn ...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could the following compounds be prepared from cyclohexanone? a. b. c. d. SCH3 ,
-
How could the following compounds be prepared from a carbonyl compound with no carbon-carbon double bonds? a. b. CH CH-CHCCH2CH2CH3 C CH-CH2 CH3
-
How could each of the following compounds be prepared from a ketone and an alkyl halide? a. b. CH,CCH2CH CH-CH2 CH2CHCCH2CH3 CH3
-
Racing Investment Fund 2000 LLC was created in August 2000 to purchase, train, and race thoroughbred horses. The LLC ' s operating agreement provided for 50 membership units to be sold for an initial...
-
In light of the winner's curse, must winning bidders in auctions necessarily "lose" in the sense of paying more than the item is worth? What steps can bidders take to prosper in auctions?
-
Use work and energy to find an expression for the speed of the block in FIGURE P10.52 just before it hits the floor if (a) The coefficient of kinetic friction for the block on the table is k (b) The...
-
Dress professionally and present yourself and your deal in a professional manner.
-
In figure, show that there are wage rates and capital rental costs such that the firm is indifferent between using the wafer-handling stepper technology and the stepper technology. How does this...
-
D Question 5 3 pts One characteristic of big data is that the data intended to be used together. Was O Was Not Question 6 3 pts Can GPS results be a source of data for Marketing Research? No Yes...
-
A TDMA-based system shown in the Figure, has a total bandwidth of 12.5 MHz and contains 20 control channels with equal channel spacing of 30 kHz. Here, the area of each cell is equal to 8 km2, and...
-
In contrast to the rapid reaction shown in Problem 23.40, ethyl acetoacetate requires a temperature over 150 ?C to undergo the same kind of cleavage reaction. How can you explain the difference in...
-
The so-called Wieland?Miescher ketone is a valuable starting material used in the synthesis of steroid hormones. How might you prepare it from 1, 3-cyclohexanedione? Wieland-Miescher ketone
-
A gas at 772 mmHg and 35.0C occupies a volume of 6.85 L. Calculate its volume at STP.
-
Watch Tre'Shawn's story (The QR code is in your text) https://www.youtube.com/watch?v=smIZLtDSPhU Using Chart 3.2 in your textbook describe what typical development for a 14-year-old boy would be...
-
Q17. An insurance company charges $500 for an insurance policy against fire and theft in the home. If a home is destroyed by fire, then the insurance company will pay the homeowner $250,000. What is...
-
If y = x ( 9 x + 5 ) , compute y ' .
-
1. Print out your name and section. 2. Create a java code to find speed of a car. a. Import the required codes to allow the user to enter data. b. The formula for speed is speed=distance/time. c. Ask...
-
Complete the square for 9 x 2 - 9 0 x + y 2 + 8 1 = 0
-
Discuss the evolution of leadership theories and their impact on modern management.
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
Write the Lewis structure for each molecule or ion. a. CI 4 b. N 2 O c. SiH 4 d. Cl 2 CO
-
Write a balanced equation for each reaction. (a) (b) (c) (d) H SO, heat CH3 CH2CH-CH NaOC(CH3 3 Br Br Nal CHCH CH-CH acetone NaOH, heat CH3 CH CCH3 Br
-
For each of the following molecular formulas, determine the number of elements of unsaturation, and draw three examples. (a) C4H4Cl2 (b) C4H8O (c) C6H8O2 (d) C5H5NO2 (e) C6H3NClBr
-
Show how you would prepare cyclopentene from each compound. (a) Trans-1, 2-dibromocyclopentane (b) Cyclopentanol (c) Cyclopentyl bromide (d) Cyclopentane (not by dehydrogenation)
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


