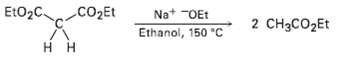
In contrast to the rapid reaction shown in Problem 23.40, ethyl acetoacetate requires a temperature over 150
Question:
In contrast to the rapid reaction shown in Problem 23.40, ethyl acetoacetate requires a temperature over 150 ?C to undergo the same kind of cleavage reaction. How can you explain the difference in reactivity?
Transcribed Image Text:
EtO2C CO2Et Na* "OEt Ethanol, 150 C 2 CH3CO2Et H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Two different reactions are possible when ethyl acetoacetate reacts with etho...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In contrast to the reaction with dilute alkali (Section 18.6), when concentrated solutions of NaOH are used, acetoacetic esters undergo cleavage as shown below.
-
In contrast to the United States, Japan has realized continuous current account surpluses. What could be the main causes for these surpluses? Is it desirable to have continuous current account...
-
In contrast to the capital asset pricing model, arbitrage pricing theory: a. Requires that markets be in equilibrium. b. Uses risk premiums based on micro variables. c. Specifies the number and...
-
A Monica who is aged 54, is employed as a Senior Manager with Abacus, a large accounting firm based in Sydney. During the 2020 tax year Abacus contributed $20,000 into QRest Super, which Monica...
-
Two incompatible high-resolution audio formats, Super Audio CD (SACD) and DVD Audio (DVDA), were introduced in 2000. Both offer surround-sound music at a quality that approaches the original studio...
-
a. In FIGURE EX10.27, what minimum speed does a 100 g particle need at point A to reach point B? b. What minimum speed does a 100 g particle need at point B to reach point A? U (J) 5- 2- 0+ B FIGURE...
-
Use proven selling skills: State the benefits of your venture, address any objections raised, and close the deal.
-
Fill in the spreadsheet below and answer the following questions. Calculate answers to three decimal places. a. Calculate Total Returns (TRs) and Return Relatives (RRs) for McDonald's for the 10...
-
A _____ current asset investment policy calls for relatively large amounts of current assets to be carried by a company. Select one: a. relaxed b. restricted c. moderate d. lean-and-mean e....
-
You are the vice president of marketing for a small software company that has developed new and novel spam-blocking software. You are charged with selecting the target market for the product launch....
-
Ethyl dimethylacetoacetate reacts instantly at room temperature when treated with ethoxide ion to yield two products, ethyl acetate and ethyl 2-mcthylpropanoatc. Propose a mechanism for this...
-
How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase. ", , (b) . (a) CHCCH-CH2CH2CH3 CH2H2CCgHs o2Et NO2 (d)...
-
Walt is single, age 67, and retired. His taxable income for 2019 is $1,320, and the tax on this amount is $132. Walt's tax credit for the elderly is $225, What is the amount of the credit for the...
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
Define leadership and identify the similarities and differences between leadership and management.
-
Subprime loans have higher loss rates than many other types of loans. Explain why lenders offer subprime loans. Describe the characteristics of the typical borrower in a subprime consumer loan.
-
Write the Lewis structure for each molecule or ion. a. N 2 H 2 b. N 2 H 4 c. C 2 H 2 d. C 2 H 4
-
Determine which compounds show cis-trans isomerism. Draw and label the isomers, using both the cis-trans and E-Z nomenclatures where applicable. (a) pent-1-ene (b) pent-2-ene (c) hex-3-ene (d)...
-
For each alkene, indicate the direction of the dipole moment. For each pair, determine which compound has the larger dipole moment. (a) Cis-1,2-difluoroethene or trans-1,2-difluoroethene (b)...
-
Predict the products of the following reactions. When more than one product is expected, predict which will be the major product. (a) (b) (c) (d) OH H2SO4 heat H3PO4 heat BrNaocH, CH CH H,SO4 heat OH
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


