Consider the following sequence of reactions, and identify the structures of compounds A, B, and C. Mg
Question:
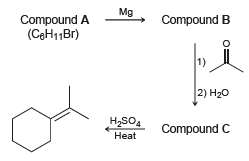
Transcribed Image Text:
Mg Compound A (CeH11Br) Compound B 1) 2) H20 H,SO4 Compound C Heat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
Br Compound ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following sequence of reactions, and identify the reagents ah. H. - ob-o - Br
-
Consider the following sequence of reactions: a) Explain how you could use IR spectroscopy to differentiate between compounds F and G. b) Explain how you could use IR spectroscopy to differentiate...
-
The herbicide trifluralin is prepared by the following sequence of reactions. Identify compound A and deduce the structure of trifluralin.
-
Consider a model of random interest rates R; between year i and i+1. The R, are i.i.d. random variables such that 1+ R; is LogNormal (0.03,0.0010) distributed. Suppose you deposit an amount of 4000...
-
Ocean Research of San Diego, California, just received a check in the amount of $800,000 from a customer in Bangor, Maine. If the firm processes the check in the normal manner, the funds will become...
-
Use a computer algebra system to graph the surface and locate any relative extrema and saddle points. (x, y) = y -3yx 2 - 3y - 3x + 1
-
Identify the adjustments made to the approximate price level on the basis of discounts, allowances, and geography.
-
The manager of a small post office is concerned that the growing township is overloading the one- window service being offered. Sample data are collected on 100 individuals who arrive for service:...
-
Do in 5 mins please From the following particulars, calculate the Fixed Overhead Expenditure variance and Fixed Overhead Volume Variance and variety their correctness : Fixed Overhead Budget for...
-
Repeat Prob. 2.75 for the eight-way divider shown in Fig. 2.136 . Prob 2.75 Find R ab in the four-way power divider circuit in Fig. 2.135. Assume each R = 4 Ω. inIim LinLw Lui bo ww- ww-...
-
A gas is slightly above its Boyle temperature. Do you expect z to increase or decrease as P increases?
-
Using 2-propanol as your only source of carbon, show how you would prepare 2-methyl-2-pentanol.
-
Amoney market mutual fund bought $1 million of two-year Treasury notes six months ago. During this time, the value of the securities has increased, but for tax reasons the mutual fund wants to...
-
The Log Jamboree amusement park ride at Six Flags over Georgia consists of an approximately rectangular flume that is 6 ft wide and is constructed from fiberglass (ks = 0.002 in). In the low-velocity...
-
57'-8" 1. The building perimeter walls are 1'2" thick and the interior walls are 1'0" thick. Fig 1 and Fig 2 detail the linear feet of 1'2" -thick foundation walls. In addition, side B is 8'4" tall...
-
The Orpheus Chamber Orchestra is celebrating its 50 years as an orchestra this year. Read the following articles about its unique structure: The first Charlotte article is copied below, the rest just...
-
Which of the five strategies for adapting products and promotion for global markets does Monster Employ? 15-16. Which factors in the global marketing environment have challenged Monster's global...
-
Analysis of Current International Economic Environment in Switzerland 1. Develop a lead sentence for this section that introduces the key subsections 2. Economic Environment describe Switzerland...
-
In Problems 18, find the real solution(s), if any, of each equation. 2x 2 - 5x - 3 = 0
-
Problem 3.5 (4 points). We will prove, in steps, that rank (L) = rank(LT) for any LE Rnxm (a) Prove that rank (L) = rank (LTL). (Hint: use Problem 3.4.) (b) Use part (a) to deduce that that rank(L) =...
-
Which of the following amino acids are more likely to be found on the outside of a globular protein, and which on the inside? Explain. (a) Valine (b) Aspartic acid (c) Phenylalanine (d) Lysine
-
The chloromethylated polystyrene resin used for Merrifield solid-phase pep- tide synthesis is prepared by treatment of polystyrene with chloromethyl methyl ether and a Lewis acid catalyst. Propose a...
-
An Fmoc protecting group can be removed from an amino acid by treatment with the amine base piperidine. Propose amechanism. pKg = 23 !! CH2 -NHCHCO- HoiHdo- NaOH H3NCHCO- Co2 2 Fmoc-protected amino...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


