Consider the structure of cyclopentadiene and then answer the following questions: (a) How many sp 3 -hybridized
Question:
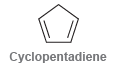
(a) How many sp3-hybridized carbon atoms are present in the structure of cyclopentadiene?
(b) Identify the most acidic proton in cyclopentadiene. Justify your choice.
(c) Draw all resonance structures of the conjugate base of cyclopentadiene.
(d) How many sp3-hybridized carbon atoms are present in the conjugate base?
(e) What is the geometry of the conjugate base?
(f) How many hydrogen atoms are present in the conjugate base?
(g) How many lone pairs are present in the conjugate base?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





